Broadly neutralizing antibodies against influenza virus and prospects for universal therapies
- PMID: 22482710
- PMCID: PMC3368890
- DOI: 10.1016/j.coviro.2012.02.005
Broadly neutralizing antibodies against influenza virus and prospects for universal therapies
Abstract
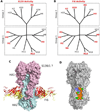
Vaccines are the gold standard for the control and prevention of infectious diseases, but a number of important human diseases remain challenging targets for vaccine development. An influenza vaccine that confers broad spectrum, long-term protection remains elusive. Several broadly neutralizing antibodies have been identified that protect against multiple subtypes of influenza A viruses, and crystal structures of several neutralizing antibodies in complex with the major influenza surface antigen, hemagglutinin, have revealed at least 3 highly conserved epitopes. Our understanding of the molecular details of these antibody-antigen interactions has suggested new strategies for the rational design of improved influenza vaccines, and has inspired the development of new antivirals for the treatment of influenza infections.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures

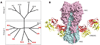
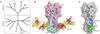
References
-
- Law M, Maruyama T, Lewis J, Giang E, Tarr AW, Stamataki Z, Gastaminza P, Chisari FV, Jones IM, Fox RI, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

