The pathophysiology of fragile X (and what it teaches us about synapses)
- PMID: 22483044
- PMCID: PMC4327822
- DOI: 10.1146/annurev-neuro-060909-153138
The pathophysiology of fragile X (and what it teaches us about synapses)
Abstract
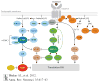
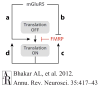
Fragile X is the most common known inherited cause of intellectual disability and autism, and it typically results from transcriptional silencing of FMR1 and loss of the encoded protein, FMRP (fragile X mental retardation protein). FMRP is an mRNA-binding protein that functions at many synapses to inhibit local translation stimulated by metabotropic glutamate receptors (mGluRs) 1 and 5. Recent studies on the biology of FMRP and the signaling pathways downstream of mGluR1/5 have yielded deeper insight into how synaptic protein synthesis and plasticity are regulated by experience. This new knowledge has also suggested ways that altered signaling and synaptic function can be corrected in fragile X, and human clinical trials based on this information are under way.
Figures






References
-
- Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–59. - PubMed
-
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Mol Cell Neurosci. 2006;32:37–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

