Solution structure analysis of the HPV16 E6 oncoprotein reveals a self-association mechanism required for E6-mediated degradation of p53
- PMID: 22483108
- PMCID: PMC3325491
- DOI: 10.1016/j.str.2012.02.001
Solution structure analysis of the HPV16 E6 oncoprotein reveals a self-association mechanism required for E6-mediated degradation of p53
Abstract
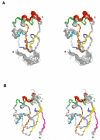
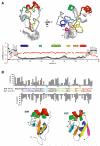
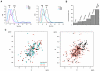
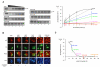
The viral oncoprotein E6 is an essential factor for cervical cancers induced by "high-risk" mucosal HPV. Among other oncogenic activities, E6 recruits the ubiquitin ligase E6AP to promote the ubiquitination and subsequent proteasomal degradation of p53. E6 is prone to self-association, which long precluded its structural analysis. Here we found that E6 specifically dimerizes through its N-terminal domain and that disruption of the dimer interface strongly increases E6 solubility. This allowed us to raise structural data covering the entire HPV16 E6 protein, including the high-resolution NMR structures of the two zinc-binding domains of E6 and a robust data-driven model structure of the N-terminal domain homodimer. Interestingly, homodimer interface mutations that disrupt E6 self-association also inactivate E6-mediated p53 degradation. These data suggest that E6 needs to self-associate via its N-terminal domain to promote the polyubiquitination of p53 by E6AP.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV, International biological study on cervical cancer (IBSCC) Study Group Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 1995;87:796–802. - PubMed
-
- Chakrabarti O, Krishna S. Molecular interactions of ‘high-risk’ human papillomaviruses E6 and E7 oncoproteins: implications for tumour progression. J. Biosci. 2003;28:337–348. - PubMed
-
- Chen JJ, Hong Y, Rustamzadeh E, Baleja JD, Androphy EJ. Identification of an alpha helical motif sufficient for association with papillomavirus E6. J. Biol. Chem. 1998;273:13537–13544. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

