The ubiquitin ligase Siah1 controls ELL2 stability and formation of super elongation complexes to modulate gene transcription
- PMID: 22483617
- PMCID: PMC3360964
- DOI: 10.1016/j.molcel.2012.03.007
The ubiquitin ligase Siah1 controls ELL2 stability and formation of super elongation complexes to modulate gene transcription
Abstract
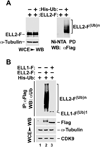
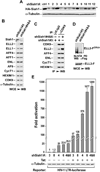
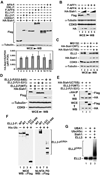
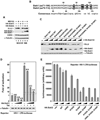
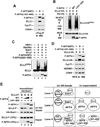
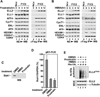
Super elongation complexes (SECs) contain two different transcription elongation factors, P-TEFb and ELL1/2, linked by the scaffolding protein AFF4 or AFF1. They stimulate the expression of both normal and disease-related genes, especially those of HIV or those involved in leukemogenesis. Among all SEC subunits, ELL2 is stoichiometrically limiting and uniquely regulated at the level of protein stability. Here we identify the RING domain protein Siah1, but not the homologous Siah2, as the E3 ubiquitin ligase for ELL2 polyubiquitination and proteasomal degradation. Siah1 cannot access and ubiquitinate ELL2 bound to AFF4, although, at high concentrations, it also degrades AFF4/1 to destroy SECs. Prostratin and HMBA, two well-studied activators of HIV transcription and latency, enhance ELL2 accumulation and SECs formation largely through decreasing Siah1 expression and ELL2 polyubiquitination. Given its importance in formation of SECs, the Siah1 ubiquitination pathway provides a fresh avenue for developing strategies to control disease-related transcription.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Apcher GS, Heink S, Zantopf D, Kloetzel PM, Schmid HP, Mayer RJ, Kruger E. Human immunodeficiency virus-1 Tat protein interacts with distinct proteasomal alpha and beta subunits. FEBS letters. 2003;553:200–204. - PubMed
-
- Bursen A, Moritz S, Gaussmann A, Dingermann T, Marschalek R. Interaction of AF4 wild-type and AF4.MLL fusion protein with SIAH proteins: indication for t(4;11) pathobiology? Oncogene. 2004;23:6237–6249. - PubMed
-
- Carthew RW, Rubin GM. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990;63:561–577. - PubMed
-
- Chin LS, Vavalle JP, Li L. Staring, a novel E3 ubiquitin-protein ligase that targets syntaxin 1 for degradation. J Biol Chem. 2002;277:35071–35079. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

