Optogenetic analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors
- PMID: 22483941
- PMCID: PMC3350619
- DOI: 10.1016/j.cub.2012.02.066
Optogenetic analysis of a nociceptor neuron and network reveals ion channels acting downstream of primary sensors
Abstract
Background: Nociception generally evokes rapid withdrawal behavior in order to protect the tissue from harmful insults. Most nociceptive neurons responding to mechanical insults display highly branched dendrites, an anatomy shared by Caenorhabditis elegans FLP and PVD neurons, which mediate harsh touch responses. Although several primary molecular nociceptive sensors have been characterized, less is known about modulation and amplification of noxious signals within nociceptor neurons. First, we analyzed the FLP/PVD network by optogenetics and studied integration of signals from these cells in downstream interneurons. Second, we investigated which genes modulate PVD function, based on prior single-neuron mRNA profiling of PVD.
Results: Selectively photoactivating PVD, FLP, and downstream interneurons via Channelrhodopsin-2 (ChR2) enabled the functional dissection of this nociceptive network, without interfering signals by other mechanoreceptors. Forward or reverse escape behaviors were determined by PVD and FLP, via integration by command interneurons. To identify mediators of PVD function, acting downstream of primary nocisensor molecules, we knocked down PVD-specific transcripts by RNAi and quantified light-evoked PVD-dependent behavior. Cell-specific disruption of synaptobrevin or voltage-gated Ca(2+) channels (VGCCs) showed that PVD signals chemically to command interneurons. Knocking down the DEG/ENaC channel ASIC-1 and the TRPM channel GTL-1 indicated that ASIC-1 may extend PVD's dynamic range and that GTL-1 may amplify its signals. These channels act cell autonomously in PVD, downstream of primary mechanosensory molecules.
Conclusions: Our work implicates TRPM channels in modifying excitability of and DEG/ENaCs in potentiating signal output from a mechano-nociceptor neuron. ASIC-1 and GTL-1 homologs, if functionally conserved, may denote valid targets for novel analgesics.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
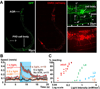




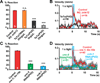

References
-
- O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 2005;8:43–50. - PubMed
-
- Tobin DM, Bargmann CI. Invertebrate nociception: behaviors, neurons and molecules. J. Neurobiol. 2004;61:161–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 MH064913/MH/NIMH NIH HHS/United States
- T32 MH64913/MH/NIMH NIH HHS/United States
- P30 EY08126/EY/NEI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- F31 NS49743/NS/NINDS NIH HHS/United States
- P60 DK20593/DK/NIDDK NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- P30 DK58404/DK/NIDDK NIH HHS/United States
- P60 DK020593/DK/NIDDK NIH HHS/United States
- R21 NS066882/NS/NINDS NIH HHS/United States
- F31 NS049743/NS/NINDS NIH HHS/United States
- R01 NS026115/NS/NINDS NIH HHS/United States
- P30 CA68485/CA/NCI NIH HHS/United States
- R21 NS06882/NS/NINDS NIH HHS/United States
- R01 NS26115/NS/NINDS NIH HHS/United States
- P01 HL6744/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

