Growth hormone secretagogue receptor (GHS-R1a) knockout mice exhibit improved spatial memory and deficits in contextual memory
- PMID: 22484009
- PMCID: PMC3361606
- DOI: 10.1016/j.bbr.2012.03.012
Growth hormone secretagogue receptor (GHS-R1a) knockout mice exhibit improved spatial memory and deficits in contextual memory
Abstract
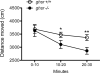
Although the hormone ghrelin is best known for its stimulatory effect on appetite and regulation of growth hormone release, it is also reported to have beneficial effects on learning and memory formation in mice. Nevertheless, controversy exists about whether endogenous ghrelin acts on its receptors in extra-hypothalamic areas of the brain. The ghrelin receptor (GHS-R1a) is co-expressed in neurons that express dopamine receptor type-1 (DRD1a) and type-2 (DRD2), and we have shown that a subset of GHS-R1a, which are not occupied by the agonist (apo-GHSR1a), heterodimerize with these two receptors to regulate dopamine signaling in vitro and in vivo. To determine the consequences of ghsr ablation on brain function, congenic ghsr -/- mice on the C57BL6/J background were subjected to a battery of behavioral tests. We show that the ghsr -/- mice exhibit normal balance, movement, coordination, and pain sensation, outperform ghsr +/+ mice in the Morris water maze, but show deficits in contextual fear conditioning.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
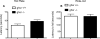
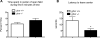
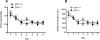
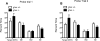
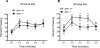

References
-
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–7. - PubMed
-
- Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, et al. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–45. - PubMed
-
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. - PubMed
-
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, et al. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

