Protein palmitoylation inhibition by 2-bromopalmitate alters gliding, host cell invasion and parasite morphology in Toxoplasma gondii
- PMID: 22484029
- PMCID: PMC3358525
- DOI: 10.1016/j.molbiopara.2012.03.006
Protein palmitoylation inhibition by 2-bromopalmitate alters gliding, host cell invasion and parasite morphology in Toxoplasma gondii
Abstract
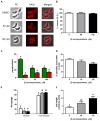
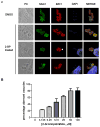
Protein palmitoylation is the reversible covalent attachment of palmitic acid onto proteins. This post-translational modification has been shown to play a part in diverse processes such as signal transduction, cellular localization and regulation of protein activity. Although many aspects of protein palmitoylation have been identified in mammalian and yeast cells, little is known of this modification in Toxoplasma gondii. In order to determine the functional role of protein palmitoylation in T. gondii, tachyzoites were treated with the palmitoylation inhibitor 2-bromopalmitate (2-BP). Parasites treated with 2-BP displayed a significant increase in non-circular trails which were longer than those trails left by non-treated parasites. Furthermore, 2-BP treatment reduced the invasion process to the host cells. Long-term treatment of intracellular tachyzoites resulted in major changes in parasite morphology and shape in a dose-dependent manner. These results suggest that palmitoylation could be modifying proteins that are key players in gliding, invasion and cytoskeletal proteins in T. gondii.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
References
-
- Linder ME, Deschenes RJ. New insights into the mechanisms of protein palmitoylation. Biochemistry. 2003;42:4311–20. - PubMed
-
- Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–83. - PubMed
-
- Corvi MM, Soltys CL, Berthiaume LG. Regulation of mitochondrial carbamoyl-phosphate synthetase 1 activity by active site fatty acylation. J Biol Chem. 2001;276:45704–12. - PubMed
-
- Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

