Intestinal redox biology and oxidative stress
- PMID: 22484611
- PMCID: PMC3396776
- DOI: 10.1016/j.semcdb.2012.03.014
Intestinal redox biology and oxidative stress
Abstract
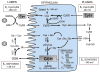
The intestinal epithelium sits at the interface between an organism and its luminal environment, and as such is prone to oxidative damage induced by luminal oxidants. Mucosal integrity is maintained by the luminal redox status of the glutathione/glutathione disulfide (GSH/GSSG) and cysteine/cystine (Cys/CySS) couples which also support luminal nutrient absorption, mucus fluidity, and a diverse microbiota. The epithelial layer is uniquely organized for rapid self-renewal that is achieved by the well-regulated processes of crypt stem cell proliferation and crypt-to-villus cell differentiation. The GSH/GSSG and Cys/CySS redox couples, known to modulate intestinal cell transition through proliferation, differentiation or apoptosis, could govern the regenerative potential of the mucosa. These two couples, together with that of the thioredoxin/thioredoxin disulfide (Trx/TrxSS) couple are the major intracellular redox systems, and it is proposed that they each function as distinctive redox control nodes or circuitry in the control of metabolic processes and networks of enzymatic reactions. Specificity of redox signaling is accomplished in part by subcellular compartmentation of the individual redox systems within the mitochondria, nucleus, endoplasmic reticulum, and cytosol wherein each defined redox environment is suited to the specific metabolic function within that compartment. Mucosal oxidative stress would result from the disruption of these unique redox control nodes, and the subsequent alteration in redox signaling can contribute to the development of degenerative pathologies of the intestine, such as inflammation and cancer.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing the paper.
Figures


References
-
- Lipkin M. Proliferation and differentiation of gastrointestinal cells. Physiol Rev. 1973;53:891–915. - PubMed
-
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. - PubMed
-
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974;141:503–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

