Nuclear factor kappa B activation occurs in the amnion prior to labour onset and modulates the expression of numerous labour associated genes
- PMID: 22485186
- PMCID: PMC3317641
- DOI: 10.1371/journal.pone.0034707
Nuclear factor kappa B activation occurs in the amnion prior to labour onset and modulates the expression of numerous labour associated genes
Abstract
Background: Prior to the onset of human labour there is an increase in the synthesis of prostaglandins, cytokines and chemokines in the fetal membranes, particular the amnion. This is associated with activation of the transcription factor nuclear factor kappa B (NFκB). In this study we characterised the level of NFκB activity in amnion epithelial cells as a measure of amnion activation in samples collected from women undergoing caesarean section at 39 weeks gestation prior to the onset of labour.
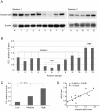
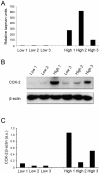
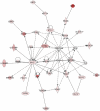
Methodology/principal findings: We found that a proportion of women exhibit low or moderate NFκB activity while other women exhibit high levels of NFκB activity (n = 12). This activation process does not appear to involve classical pathways of NFκB activation but rather is correlated with an increase in nuclear p65-Rel-B dimers. To identify the full range of genes upregulated in association with amnion activation, microarray analysis was performed on carefully characterised non-activated amnion (n = 3) samples and compared to activated samples (n = 3). A total of 919 genes were upregulated in response to amnion activation including numerous inflammatory genes such cyclooxygenase-2 (COX-2, 44-fold), interleukin 8 (IL-8, 6-fold), IL-1 receptor accessory protein (IL-1RAP, 4.5-fold), thrombospondin 1 (TSP-1, 3-fold) and, unexpectedly, oxytocin receptor (OTR, 24-fold). Ingenuity Pathway Analysis of the microarray data reveal the two main gene networks activated concurrently with amnion activation are i) cell death, cancer and morphology and ii) cell cycle, embryonic development and tissue development.
Conclusions/significance: Our results indicate that assessment of amnion NFκB activation is critical for accurate sample classification and subsequent interpretation of data. Collectively, our data suggest amnion activation is largely an inflammatory event that occurs in the amnion epithelial layer as a prelude to the onset of labour.
Conflict of interest statement
Figures

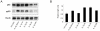

References
-
- Okita JR, MacDonald PC, Johnston JM. Mobilization of arachidonic acid from specific glycerophospholipids of human fetal membranes during early labor. J Biol Chem. 1982;257:14029–14034. - PubMed
-
- Okita JR, Sagawa N, Casey ML, Snyder JM. A comparison of human amnion tissue and amnion cells in primary culture by morphological and biochemical criteria. In Vitro. 1983;19:117–126. - PubMed
-
- Bennett PR, Elder MG. The mechanisms of preterm labour; the interaction between amnion cells and leukocytes in the metabolism of arachidonic acid. Prostaglandins. 1992;43:87–98. - PubMed
-
- Sangha RK, Walton JC, Ensor CM, Tai HH, Challis JR. Immunohistochemical localization, messenger ribonucleic acid abundance, and activity of 15-hydroxyprostaglandin dehydrogenase in placenta and fetal membranes during term and preterm labor. J Clin Endocrinol Metab. 1994;78:982–989. - PubMed
-
- Sadovsky Y, Nelson DM, Muglia LJ, Gross GA, Harris KC, et al. Effective diminution of amniotic prostaglandin production by selective inhibitors of cyclooxygenase type 2. Am J Obstet Gynecol. 2000;182:370–376. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

