Ethanol metabolism and osmolarity modify behavioral responses to ethanol in C. elegans
- PMID: 22486589
- PMCID: PMC3396773
- DOI: 10.1111/j.1530-0277.2012.01799.x
Ethanol metabolism and osmolarity modify behavioral responses to ethanol in C. elegans
Abstract
Background: Ethanol (EtOH) is metabolized by a 2-step process in which alcohol dehydrogenase (ADH) oxidizes EtOH to acetaldehyde, which is further oxidized to acetate by aldehyde dehydrogenase (ALDH). Although variation in EtOH metabolism in humans strongly influences the propensity to chronically abuse alcohol, few data exist on the behavioral effects of altered EtOH metabolism. Here, we used the nematode Caenorhabditis elegans to directly examine how changes in EtOH metabolism alter behavioral responses to alcohol during an acute exposure. Additionally, we investigated EtOH solution osmolarity as a potential explanation for contrasting published data on C. elegans EtOH sensitivity.
Methods: We developed a gas chromatography assay and validated a spectrophotometric method to measure internal EtOH in EtOH-exposed worms. Further, we tested the effects of mutations in ADH and ALDH genes on EtOH tissue accumulation and behavioral sensitivity to the drug. Finally, we tested the effects of EtOH solution osmolarity on behavioral responses and tissue EtOH accumulation.
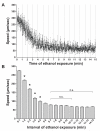
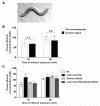
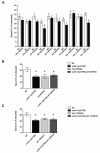
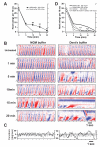
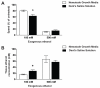
Results: Only a small amount of exogenously applied EtOH accumulated in the tissues of C. elegans and consequently their tissue concentrations were similar to those that intoxicate humans. Independent inactivation of an ADH-encoding gene (sodh-1) or an ALDH-encoding gene (alh-6 or alh-13) increased the EtOH concentration in worms and caused hypersensitivity to the acute sedative effects of EtOH on locomotion. We also found that the sensitivity to the depressive effects of EtOH on locomotion is strongly influenced by the osmolarity of the exogenous EtOH solution.
Conclusions: Our results indicate that EtOH metabolism via ADH and ALDH has a statistically discernable but surprisingly minor influence on EtOH sedation and internal EtOH accumulation in worms. In contrast, the osmolarity of the medium in which EtOH is delivered to the animals has a more substantial effect on the observed sensitivity to EtOH.
Copyright © 2012 by the Research Society on Alcoholism.
Figures
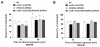
References
-
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. - PubMed
-
- Bettinger JC, McIntire SL. State-dependency in C. elegans. Genes Brain Behav. 2004;3:266–272. - PubMed
-
- Burns AR, Wallace IM, Wildenhain J, Tyers M, Giaever G, Bader GD, Nislow C, Cutler SR, Roy PJ. A predictive model for drug bioaccumulation and bioactivity in Caenorhabditis elegans. Nat Chem Biol. 2010;6:549–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AA007471/AA/NIAAA NIH HHS/United States
- 5T32AA007471/AA/NIAAA NIH HHS/United States
- R01 AA016842/AA/NIAAA NIH HHS/United States
- R01AA016842/AA/NIAAA NIH HHS/United States
- R01 AA020634/AA/NIAAA NIH HHS/United States
- R01 AA016837/AA/NIAAA NIH HHS/United States
- R01 NS075541/NS/NINDS NIH HHS/United States
- P20 AA017828/AA/NIAAA NIH HHS/United States
- R01AA016837/AA/NIAAA NIH HHS/United States
- R03AA020195/AA/NIAAA NIH HHS/United States
- R03 AA020195/AA/NIAAA NIH HHS/United States
- P20AA017070/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources

