OSVZ progenitors in the human cortex: an updated perspective on neurodevelopmental disease
- PMID: 22487088
- PMCID: PMC3402619
- DOI: 10.1016/j.conb.2012.03.006
OSVZ progenitors in the human cortex: an updated perspective on neurodevelopmental disease
Abstract
Recent discoveries concerning the architecture and cellular dynamics of the developing human brain are revealing new differences between mouse and human cortical development. In mice, neurons are produced by ventricular radial glial (RG) cells and subventricular zone intermediate progenitor (IP) cells. In the human cortex, both ventricular RG and highly motile outer RG cells generate IP cells, which undergo multiple rounds of transit amplification in the outer subventricular zone before producing neurons. This creates a more complex environment for neurogenesis and neuronal migration, adding new arenas in which neurodevelopmental disease gene mutation could disrupt corticogenesis. A more complete understanding of disease mechanisms will involve use of emerging model systems with developmental programs more similar to that of the human neocortex.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures

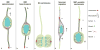
References
-
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464(7288):554–61. Using histological labeling techniques and timelapse imaging, this paper describes the progenitor cell composition of the developing human neocortex. The authors show that a novel class of radial glia found in the OSVZ, termed oRG cells, are neural stem cells, and may be responsible for increasing neuronal number and cortical gyration in evolution. - PubMed
-
- Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, Huttner WB. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13(6):690–9. This paper describes the presence of oRG cells in the human, and extends analysis of oRG cells analysis to another gyrencephalic mammal, the ferret. This is the first evidence that oRG cells are not specific to humans or primates, and may represent a more general mechanism for cortical expansion in many species. - PubMed
-
- Kelava I, Reillo I, Murayama AY, Kalinka AT, Stenzel D, Tomancak P, Matsuzaki F, Lebrand C, Sasaki E, Schwamborn JC, Okano H, Huttner WB, Borrell V. Abundant Occurrence of Basal Radial Glia in the Subventricular Zone of Embryonic Neocortex of a Lissencephalic Primate, the Common Marmoset Callithrix jacchus. Cereb Cortex. 2012;22(2):469–81. - PMC - PubMed
-
- Garcia-Moreno F, Vasistha NA, Trevia N, Bourne JA, Molnar Z. Compartmentalization of cerebral cortical germinal zones in a lissencephalic primate and gyrencephalic rodent. Cereb Cortex. 2012;22(2):482–92. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

