A new model of Pde4d deficiency: genetic knock-down of PDE4D enzyme in rats produces an antidepressant phenotype without spatial cognitive effects
- PMID: 22487514
- PMCID: PMC4511101
- DOI: 10.1111/j.1601-183X.2012.00796.x
A new model of Pde4d deficiency: genetic knock-down of PDE4D enzyme in rats produces an antidepressant phenotype without spatial cognitive effects
Abstract
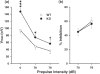
Phosphodiesterases (PDEs) are a superfamily of intracellular second messenger cyclic nucleotide hydrolyzing enzymes composed of 12 families. The Pde4 family has been implicated in depression and cognition, and PDE4 inhibitors have been evaluated as antidepressants and possible cognitive enhancers. Pde4d(-/-) mice show an antidepressant phenotype and learning enhancement on some tests, but not others as do mice treated with PDE4 inhibitors. Here, we report for the first time the behavioral phenotype of a new Pde4d knock-down (KD) rat model of PDE4D deficiency. Consistent with other data on PDE4D deficiency, Pde4d KD rats showed depression resistance in the Porsolt forced swim test and hyperreactivity of the acoustic startle response with no differential response on prepulse inhibition, suggesting no sensorimotor gating defect. Pde4d KD rats also exhibited a small exploratory activity reduction but no difference following habituation, and no enhanced spatial learning or reference memory in the Morris water maze. A selective improvement in route-based learning in the Cincinnati water maze was seen as well as enhanced contextual and cued fear conditioning and a more rapid rate of cued extinction from their higher freezing level that declined to wild-type (WT) levels only after ∼20 extinction trials. The rat model confirms Pde4d's role in depression but not in spatial learning or memory enhancement and shows for the first time higher fear conditioning and altered extinction compared with controls. The new model provides a tool by which to better understand the role of PDE4D in neuropsychiatric disorders and for the development of alternate treatment approaches.
© 2012 The Authors. Genes, Brain and Behavior © 2012 Blackwell Publishing Ltd and International Behavioural and Neural Genetics Society.
Conflict of interest statement
TLS, AAB, RMAK, MTW, and CVV declare no conflict of interest. EO is President and CEO of Transposagen Biopharmaceutical Company that provided the gene-targeted animals for this project.
Figures

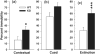
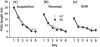


References
-
- Bobon D, Breulet M, Gerard-Vandenhove MA, Guiot-Goffioul F, Plomteux G, Hernandez M, Schratzer M, Troisfontaines B, von FR, Wachtel H. Is phosphodiesterase inhibition a new mechanism of antidepressant action? A double blind double-dummy study between rolipram and desipramine in hospitalized major and/or endogenous depressives. Eur Arch Psychiatry Neurol Sci. 1988;238:2–6. - PubMed
-
- Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, Thorsteinsdottir M, Hrafnsdottir S, Hagen T, Kiselyov AS, Stewart LJ, Gurney ME. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70. - PubMed
-
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

