Clinical outcomes of percutaneous interventions in saphenous vein grafts using drug-eluting stents compared to bare-metal stents: a comprehensive meta-analysisof all randomized clinical trials
- PMID: 22488047
- PMCID: PMC6652335
- DOI: 10.1002/clc.21984
Clinical outcomes of percutaneous interventions in saphenous vein grafts using drug-eluting stents compared to bare-metal stents: a comprehensive meta-analysisof all randomized clinical trials
Abstract
Background: Clinical outcomes of percutaneous coronary intervention (PCI) in patients with saphenous vein grafts (SVGs) remain poor despite the use of drug-eluting stents (DES). There is a disparity in clinical outcomes in SVG PCI based on various registries, and randomized clinical data remain scant. We conducted a meta-analysis of all existing randomized controlled trials (RCTS) comparing bare-metal stents (BMS) and DES in SVGPCIs.
Hypothesis: PCI in patients with SVG disease using DES may reduce need for repeat revascularization without an excess mortality when compared to BMS.
Methods: An aggregate data meta-analysis of clinical outcomes in RCTs comparing PCI with DES vs BMS for SVGs reporting at least 12 months of follow-up was performed. A literature search between Janurary 1, 2003 and September 30, 2011 identified 4 RCTs (812 patients; DES = 416, BMS = 396). Summary odds ratio (OR) and 95% confidence interval (CI) were calculated using the random-effects model. The primary endpoint was all-cause mortality. Secondary outcomes included nonfatal myocardial infarction (MI), repeat revascularization, and major adverse cardiac events (MACE). These outcomes were assessed in a cumulative fashion at 30 days, 18 months, and 36 months.
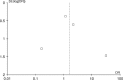
Results: There were no intergroup differences in baseline clinical and sociodemographic characteristics. At a median follow-up of 25 months, patients in the DES and BMS group had similar rates of death (OR: 1.63, 95% CI: 0.45-5.92), MI (OR; 0.83, 95% CI: 0.27-2.60), and MACE (OR: 0.58, 95% CI: 0.25-1.32). Patients treated with DES had lower rates of repeat revascularization (OR: 0.40, 95% CI: 0.22-0.75).
Conclusions: In this comprehensive meta-analysis of all RCTs comparing clinical outcomes of PCI using DES vs BMS in patients with SVG disease, use of DES was associated with a reduction in rate of repeat revascularization and no difference in rates of all-cause death and MI. Clin. Cardiol. 2012 DOI: 10.1002/clc.21984 Dr. Virani is supported by a Department of Veterans Affairs Health Services Research and Development Service (HSR&D) Career Development Award (CDA-09-028), and has research support from Merck and National Football League Charities (all grants to the institution and not individual). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
© 2012 Wiley Periodicals, Inc.
Figures

References
-
- Coolong A, Baim DS, Kuntz RE, et al. Saphenous vein graft stenting and major adverse cardiac events: a predictive model derived from a pooled analysis of 3958 patients. Circulation. 2008;117:790–797. - PubMed
-
- Hakeem A, Helmy T, Munsif S, et al. Safety and efficacy of drug eluting stents compared with bare metal stents for saphenous vein graft interventions: a comprehensive meta‐analysis of randomized trials and observational studies comprising 7,994 patients. Catheter CardiovascInterv. 2011;77:343–355. - PubMed
-
- Nwasokwa ON. Coronary artery bypass graft disease. Ann Intern Med. 1995. 1;123:528–545. - PubMed
-
- Brilakis ES, Wang TY, Rao SV, et al. Frequency and Predictors of drug‐eluting stent use in saphenous vein bypass graft percutaneous coronary interventions: a report from the American College of Cardiology National Cardiovascular Data Cath PCI registry. JACC Cardio vascInterv. 2010;3:1068–1073. - PubMed
-
- Mamas MA, Foley J, Nair S, et al. A comparison of drug‐eluting stents versus bare metal stents in saphenous vein graft PCI outcomes: a meta‐analysis. J IntervCardiol. 2011;24:172–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

