Distinct contribution of human cord blood-derived endothelial colony forming cells to liver and gut in a fetal sheep model
- PMID: 22488442
- PMCID: PMC3396735
- DOI: 10.1002/hep.25753
Distinct contribution of human cord blood-derived endothelial colony forming cells to liver and gut in a fetal sheep model
Abstract
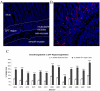
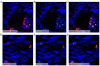
Although the vasculogenic potential of circulating and cord blood (CB)-derived endothelial colony-forming cells (ECFC) has been demonstrated in vitro and in vivo, little is known about the inherent biologic ability of these cells to home to different organs and contribute to tissue-specific cell populations. Here we used a fetal sheep model of in utero transplantation to investigate and compare the intrinsic ability of human CB-derived ECFC to migrate to the liver and to the intestine, and to define ECFC's intrinsic ability to integrate and contribute to the cytoarchitecture of these same organs. ECFCs were transplanted by an intraperitoneal or intrahepatic route (IH) into fetal sheep at concentrations ranging from 1.1-2.6 × 10(6) cells/fetus. Recipients were evaluated at 85 days posttransplant for donor (human) cells using flow cytometry and confocal microscopy. We found that, regardless of the route of injection, and despite the IH delivery of ECFC, the overall liver engraftment was low, but a significant percentage of cells were located in the perivascular regions and retained the expression of hallmark endothelial makers. By contrast, ECFC migrated preferentially to the intestinal crypt region and contributed significantly to the myofibroblast population. Furthermore, ECFC expressing CD133 and CD117 lodged in areas where endogenous cells expressed those same phenotypes.
Conclusion: ECFC inherently constitute a potential source of cells for the treatment of intestinal diseases, but strategies to increase the numbers of ECFC persisting within the hepatic parenchyma are needed in order to enhance ECFC therapeutic potential for this organ.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures





References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, et al. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275:964–966. - PubMed
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, et al. Bone Marrow Origin of Endothelial Progenitor Cells Responsible for Postnatal Vasculogenesis in Physiological and Pathological Neovascularization. Circulation Research. 1999;85:221–228. - PubMed
-
- Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29:1650–1655. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
