Compensatory redistribution of neuroligins and N-cadherin following deletion of synaptic β1-integrin
- PMID: 22488504
- PMCID: PMC3496382
- DOI: 10.1002/cne.23027
Compensatory redistribution of neuroligins and N-cadherin following deletion of synaptic β1-integrin
Abstract
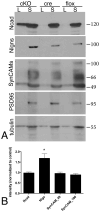
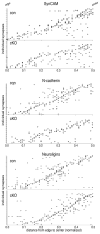
β1-containing integrins are required for persistent synaptic potentiation in hippocampus and regulate hippocampal-dependent learning. Based largely on indirect evidence, there is a prevailing assumption that β1-integrins are localized at synapses, where they contribute to synapse adhesion and signaling, but this has not been examined directly. Here we investigate the fine localization of β1-integrin in adult mouse hippocampus using high-resolution immunogold labeling, with a particular emphasis on synaptic labeling patterns. We find that β1-integrins localize to synapses in CA1 and are concentrated postsynaptically. At the postsynaptic membrane, β1-integrins are found more commonly clustered near active zone centers rather than at the peripheral edges. In mice harboring a conditional deletion of β1-integrins, labeling for N-cadherin and neuroligins increases. Western blots show increased levels of N-cadherin in total lysates and neuroligins increase selectively in synaptosomes. These data suggest there is a dynamic, compensatory adjustment of synaptic adhesion. Such adjustment is specific only for certain cell adhesion molecules (CAMs), because labeling for SynCAM is unchanged. Together, our findings demonstrate unequivocally that β1-integrin is an integral synaptic adhesion protein, and suggest that adhesive function at the synapse reflects a cooperative and dynamic network of multiple CAM families.
Copyright © 2011 Wiley Periodicals, Inc.
Figures


Similar articles
-
N-cadherin and neuroligins cooperate to regulate synapse formation in hippocampal cultures.J Biol Chem. 2011 Jan 7;286(1):851-8. doi: 10.1074/jbc.M110.176305. Epub 2010 Nov 5. J Biol Chem. 2011. PMID: 21056983 Free PMC article.
-
Immunoelectron microscopic localization of the neural recognition molecules L1, NCAM, and its isoform NCAM180, the NCAM-associated polysialic acid, beta1 integrin and the extracellular matrix molecule tenascin-R in synapses of the adult rat hippocampus.J Neurobiol. 2001 Nov 5;49(2):142-58. doi: 10.1002/neu.1071. J Neurobiol. 2001. PMID: 11598921
-
Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism.Mol Psychiatry. 2017 Mar;22(3):375-383. doi: 10.1038/mp.2016.80. Epub 2016 May 24. Mol Psychiatry. 2017. PMID: 27217145 Free PMC article.
-
Neuroligins, synapse balance and neuropsychiatric disorders.Pharmacol Rep. 2014 Oct;66(5):830-5. doi: 10.1016/j.pharep.2014.04.011. Epub 2014 May 9. Pharmacol Rep. 2014. PMID: 25149987 Review.
-
Cell adhesion molecules at the synapse.Front Biosci. 2006 Sep 1;11:2400-19. doi: 10.2741/1978. Front Biosci. 2006. PMID: 16720322 Review.
Cited by
-
The Synaptic Extracellular Matrix: Long-Lived, Stable, and Still Remarkably Dynamic.Front Synaptic Neurosci. 2022 Mar 8;14:854956. doi: 10.3389/fnsyn.2022.854956. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35350469 Free PMC article. Review.
-
Soluble ICAM-5, a product of activity dependent proteolysis, increases mEPSC frequency and dendritic expression of GluA1.PLoS One. 2013 Jul 2;8(7):e69136. doi: 10.1371/journal.pone.0069136. Print 2013. PLoS One. 2013. PMID: 23844251 Free PMC article.
-
The role of the actin cytoskeleton in regulating Drosophila behavior.Rev Neurosci. 2013;24(5):471-84. doi: 10.1515/revneuro-2013-0017. Rev Neurosci. 2013. PMID: 24077615 Free PMC article. Review.
-
Maturation of cortical circuits requires Semaphorin 7A.Proc Natl Acad Sci U S A. 2014 Sep 23;111(38):13978-83. doi: 10.1073/pnas.1408680111. Epub 2014 Sep 8. Proc Natl Acad Sci U S A. 2014. PMID: 25201975 Free PMC article.
-
Activity dependent CAM cleavage and neurotransmission.Front Cell Neurosci. 2015 Aug 11;9:305. doi: 10.3389/fncel.2015.00305. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26321910 Free PMC article. Review.
References
-
- Baude A, Nusser Z, Molnár E, McIlhinney RAJ, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. - PubMed
-
- Bernard-Trifilo JA, Kramar EA, Torp R, Lin CY, Pineda EA, Lynch G, Gall CM. Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J Neurochem. 2005;93(4):834–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

