Genetic disruption of Pten in a novel mouse model of tomaculous neuropathy
- PMID: 22488882
- PMCID: PMC3443946
- DOI: 10.1002/emmm.201200227
Genetic disruption of Pten in a novel mouse model of tomaculous neuropathy
Abstract
'Tomacula' and myelin outfoldings are striking neuropathological features of a diverse group of inherited demyelinating neuropathies. Whereas the underlying genetic defects are well known, the molecular mechanisms of tomacula formation have remained obscure. We hypothesized that they are caused by uncontrolled, excessive myelin membrane growth, a process, which is regulated in normal development by neuregulin-1/ErbB2, PI3 Kinase signalling and ERK/MAPK signalling. Here, we demonstrate by targeted disruption of Pten in Schwann cells that hyperactivation of the endogenous PI3 Kinase pathway causes focal hypermyelination, myelin outfoldings and tomacula, even when induced in adult animals by tamoxifen, and is associated with progressive peripheral neuropathy. Activated AKT kinase is associated with PtdIns(3,4,5)P(3) at paranodal loops and Schmidt-Lanterman incisures. This striking myelin pathology, with features of human CMT type 4B1 and HNPP, is dependent on AKT/mTOR signalling, as evidenced by a significant amelioration of the pathology in mice treated with rapamycin. We suggest that regions of non-compact myelin are under lifelong protection by PTEN against abnormal membrane outgrowth, and that dysregulated phosphoinositide levels play a critical role in the pathology of tomaculous neuropathies.
Copyright © 2012 EMBO Molecular Medicine.
Figures

Westernblot analysis of sciatic nerve lysates obtained from two controls and two mutants at the age of 3 months. PTEN protein is decreased in the mutants and P-AKT, P-mTOR and P-S6 levels are increased. In contrast the levels of phosphorylated ERK1 and ERK2 are unchanged in mutants compared to controls.
Scatter plot depicting g-ratios (ordinate) of 1.5 month-old mutant and age-matched control mice in relation to the axon diameter (abscissa) based on semithin sections of sciatic nerves at comparable levels (left panel). Axons with calibres larger than 2 µm in diameter show no alteration in myelin sheath thickness in mutants when compared to controls (right panel) (n = 6 for mutants; n = 8 for controls; p = 0.9728). Error bars represent the SEM. The p value was calculated by two-tailed Student's t-test.
Left panels: light-microscopic analysis of myelin abnormalities on sciatic nerve transverse semithin-sections (500 nm) of mutants (Ptenflox/flox*CnpCre/+) and controls (Ptenflox/flox*Cnp+/+) at the indicated ages. Mutant nerves are characterized by a progressive development of myelin abnormalities like tomacula (arrowheads), comma-shaped myelin outfoldings (arrows) and recurrent myelin loops (asterisks). Scale bars, 20 µm. Right panels: light-microscopic analysis of sciatic nerve semithin cross-sections (500 nm) reveals no myelin pathology in heterozygous Ptenflox/+*CnpCre/+ mice when compared to Pten+/+*CnpCre/+ controls at 1 year of age.
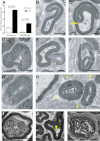
A. When counted on semithin sciatic nerve cross sections, tomacula and comma-shaped outfoldings increased in number with age (tomacula: 1.44 ± 0.10% for P12 mutants and 6.55 ± 0.07% for P90 mutants; outfoldings: 0.24 ± 0.07% for P12 mutants and 2.76 ± 0.25% for P90 mutants; n = 3 per genotype and age). Error bars represent the SEM. p values were calculated by two-tailed Student's t-test.
B–K. Ultrastructural analysis of focally redundant myelin in mutant sciatic nerves appearing as comma-shaped outfoldings (B, C), tomacula (D) and recurrent loops (arrow in E). Myelin outfoldings contain no axonal structures (arrowhead in C). Invaginating recurrent loops exhibit the same spacing and number of lamellae as the myelin sheath they originate from (inset in F). Nearly regular appearing hypermyelination also occurs (G). Occasionally, processes of macrophages are observed that actively remove redundant myelin (arrowheads in H). Accumulation of axoplasmic organelles (I), degeneration of microtubules and neurofilaments (arrowhead in J), and Wallerian-type degeneration (K) indicate disturbed axonal functions. A, axon. MD, myelin debris. Scale bars, 1 µm.

Osmicated teased nerve fibres from 1.5 month-old control and mutant mice. Focal myelin thickenings preferentially originate at paranodal regions (black arrows) and Schmidt–Lanterman incisures (SLI) (yellow arrows). SLI are indicated by asterisks. Note in left panel that either one or both paranodal regions can be affected. Scale bars, 10 µm.
Ultrastructural appearance of exuberant myelin growth (arrows) at paranodal regions (left panel) and SLI (marked by asterisk in right panel). A, axon; N, node. Scale bars, 2 µm.
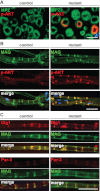
Immunostaining of sciatic nerve cross sections with antibodies against p-AKT (in red) and MPZ as a marker of compact myelin (in green). Scalebar, 10 µm.
Teased sciatic nerve fibres stained with antibodies against p-AKT (in red) and MAG as a marker of non-compact myelin (in green). Scale bars, 50 µm.
Costaining of MAG and Dlg1 (upper panels) and MAG and Par-3 (lower panels). Dlg1 and Par-3 are similarly enriched in mutants and controls in regions of non-compact myelin. Scale bar, 50 µm.

A. Representative traces of sciatic nerve CMAPs recorded from a foot muscle after proximal and distal stimulation. Arrows indicate the stimulus and the onset of the CMAP. Asterisk marks the F-wave latency. Compared to controls, Pten mutants display prolonged latencies and decreased CMAP amplitudes.
B–D. Motor nerve conduction studies of 43 days old controls and mutants (n = 3 per group). Mutants show significantly reduced CMAP amplitudes after (B) distal and (C) proximal stimulation. (D) Sciatic NCV is significantly slowed in Pten mutants compared to controls. Error bars represent the SEM. p values were calculated by two-tailed Student's t-test.

A. Westernblots of sciatic nerve lysates from mutant mice treated with placebo (−Rap) or rapamycin (+Rap) from P13 to P43. Rapamycin treatment substantially reduced downstream phosphorylation of S6 but did not lower total protein levels of S6 or levels of P-AKT when compared to Actin as a loading control.
B–F. When quantified on semithin cross sections, the total number of myelinated axons was invariably higher in mutants than in controls and not influenced by rapamycin treatment (B). While the density of myelinated axons was reduced in mutants(−Rap) when compared to controls(−Rap), rapamycin treatment significantly restored the axonal density in mutants(+Rap) closer to control levels (C). Rapamycin treatment also significantly lowered the number of tomacula (D), recurrent loops (E) and outfoldings (F) in the mutants(+Rap) when compared to mutants(−Rap). Controls(−Rap), n = 8; controls(+Rap), n = 6; mutants(−Rap), n = 6; and mutants(+Rap), n = 8. Error bars represent the SEM. p values were calculated by two-tailed Student's t-test.
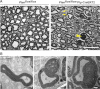
Semithin sections of a control mouse (Ptenflox/flox) and a double transgenic mutant mouse harbouring a Plp-CreERT2 transgene (Ptenflox/flox*Plp-CreERT2). Mice were treated with TM at 2 months of age and analysed 3.5 months later. Outfoldings (arrows) and tomacula (arrowhead) also occur after ablation of Pten in adult mice. Scale bar, 20 µm.
Ultrastructural analysis of myelin pathology after adult inactivation of Pten with comma-shaped outfoldings (left panel), recurrent loops (middle panel) and tomacula (right panel). Scale bars, 1 µm.
References
-
- Adey NB, Huang L, Ormonde PA, Baumgard ML, Pero R, Byreddy DV, Tavtigian SV, Bartel PL. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 2000;60:35–37. - PubMed
-
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave K-A, Franklin RJM, Meijer D, et al. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177:1051–1061. - PMC - PubMed
-
- Berger P, Niemann A, Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease) Glia. 2006;54:243–257. - PubMed
-
- Bolino A, Muglia M, Conforti FL, LeGuern E, Salih MA, Georgiou DM, Christodoulou K, Hausmanowa-Petrusewicz I, Mandich P, Schenone A, et al. Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet. 2000;25:17–19. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

