Phage display approaches for the isolation of monoclonal antibodies against dengue virus envelope domain III from human and mouse derived libraries
- PMID: 22489114
- PMCID: PMC3317677
- DOI: 10.3390/ijms13032618
Phage display approaches for the isolation of monoclonal antibodies against dengue virus envelope domain III from human and mouse derived libraries
Abstract
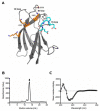
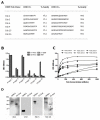
Domain III of the dengue virus envelope protein (EDIII, aa295-395) has an immunoglobulin fold and is the proposed receptor-binding domain of the virus. Previous studies have shown that monoclonal antibodies against EDIII can be neutralizing and have therapeutic potential. Here, cloned Fab-phage libraries of human and mouse origin were screened for DENV specific antibodies. Firstly, bacterially expressed EDIII or whole virus particles were used as bait in biopanning against a large naïve human Fab-phage library (>10 billion independent clones). Multiple panning strategies were employed, and in excess of 1000 clones were screened, but all of the antibodies identified bound the envelope in regions outside EDIII suggesting EDIII antibodies are virtually absent from the naïve human repertoire. Next, a chimeric Fab-phage library was constructed from a panel of EDIII specific mouse hybridomas by pooling the VH and VL chain sequences from the hybridomas and cloning these into the pComb3X phagemid vector with human CH and CL encoding sequences. Biopanning against EDIII identified a unique antibody (C9) that cross-reacts with EDIII from DENV1-3 and, in the IgG format, binds and neutralizes DENV2 in cell-based assays. Sequence analysis and saturation mutagenesis of complementary determining regions (CDR) in the C9 light chain suggest an antigen recognition model in which the LCDR3 is a key determinant of EDIII specificity, while modifications in LCDR1 and LCDR2 affect DENV serotype cross-reactivity. Overall, this study supports the current prevailing opinion that neutralizing anti-EDIII monoclonal antibodies can be readily generated in murine systems, but in humans the anti-DENV immune response is directed away from domain III.
Keywords: E protein; Fab; dengue; domain III; hybridoma; phage display.
Figures
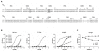

Similar articles
-
Human monoclonal single-chain antibodies specific to dengue virus envelope protein.Lett Appl Microbiol. 2014 Mar;58(3):270-7. doi: 10.1111/lam.12186. Epub 2013 Nov 25. Lett Appl Microbiol. 2014. PMID: 24266517
-
Dengue virus envelope domain III immunization elicits predominantly cross-reactive, poorly neutralizing antibodies localized to the AB loop: implications for dengue vaccine design.J Gen Virol. 2013 Oct;94(Pt 10):2191-2201. doi: 10.1099/vir.0.055178-0. Epub 2013 Jul 12. J Gen Virol. 2013. PMID: 23851440
-
An Engineered N-Glycosylated Dengue Envelope Protein Domain III Facilitates Epitope-Directed Selection of Potently Neutralizing and Minimally Enhancing Antibodies.ACS Infect Dis. 2024 Aug 9;10(8):2690-2704. doi: 10.1021/acsinfecdis.4c00058. Epub 2024 Jun 29. ACS Infect Dis. 2024. PMID: 38943594 Free PMC article.
-
Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine.Front Cell Infect Microbiol. 2020 Oct 22;10:572681. doi: 10.3389/fcimb.2020.572681. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194810 Free PMC article. Review.
-
The pComb3 Phagemid Family of Phage Display Vectors.Cold Spring Harb Protoc. 2024 Aug 1;2024(8):pdb.over107756. doi: 10.1101/pdb.over107756. Cold Spring Harb Protoc. 2024. PMID: 37460147 Review.
Cited by
-
Dengue virus antibody database: Systematically linking serotype-specificity with epitope mapping in dengue virus.PLoS Negl Trop Dis. 2017 Feb 21;11(2):e0005395. doi: 10.1371/journal.pntd.0005395. eCollection 2017 Feb. PLoS Negl Trop Dis. 2017. PMID: 28222130 Free PMC article.
-
Generation of Recombinant Antibodies Against Toxins and Viruses by Phage Display for Diagnostics and Therapy.Adv Exp Med Biol. 2016;917:55-76. doi: 10.1007/978-3-319-32805-8_4. Adv Exp Med Biol. 2016. PMID: 27236552 Free PMC article. Review.
-
Developing Recombinant Antibodies by Phage Display Against Infectious Diseases and Toxins for Diagnostics and Therapy.Front Cell Infect Microbiol. 2021 Jul 7;11:697876. doi: 10.3389/fcimb.2021.697876. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34307196 Free PMC article. Review.
-
Molecular characterization of the viral structural protein genes in the first outbreak of dengue virus type 2 in Hunan Province, inland China in 2018.BMC Infect Dis. 2021 Feb 10;21(1):166. doi: 10.1186/s12879-021-05823-3. BMC Infect Dis. 2021. PMID: 33568111 Free PMC article.
-
The Complexity of a Dengue Vaccine: A Review of the Human Antibody Response.PLoS Negl Trop Dis. 2015 Jun 11;9(6):e0003749. doi: 10.1371/journal.pntd.0003749. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26065421 Free PMC article. Review.
References
-
- Lindenbach B.D., Thiel H.J., Rice C.M. Fields Virology. 5th ed. Lippincott William; Philadelphia, PA, USA: 2007. Flaviviridae: the viruses and their replication; pp. 1101–1151.
-
- Halstead S.B. Dengue. Lancet. 2007;370:1644–1652. - PubMed
-
- Fink J., Gu F., Vasudevan S.G. Role of T cells, cytokines and antibody in dengue fever and dengue haemorrhagic fever. Rev. Med. Virol. 2006;16:263–275. - PubMed
-
- Falconar A.K. Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch. Virol. 1999;144:2313–2330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

