Photosensized controlling benzyl methacrylate-based matrix enhanced Eu(3+) narrow-band emission for fluorescence applications
- PMID: 22489178
- PMCID: PMC3317738
- DOI: 10.3390/ijms13033718
Photosensized controlling benzyl methacrylate-based matrix enhanced Eu(3+) narrow-band emission for fluorescence applications
Abstract
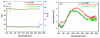
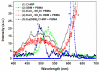
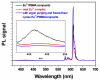
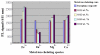
This study synthesized a europium (Eu(3+)) complex Eu(DBM)(3)Cl-MIP (DBM = dibenzoyl methane; Cl-MIP = 2-(2-chlorophenyl)-1-methyl-1H-imidazo[4,5-f][1,10]phenanthroline) dispersed in a benzyl methacrylate (BMA) monomer and treated with ultraviolet (UV) light for polymerization. Spectral results showed that the europium complex containing an antenna, Cl-MIP, which had higher triplet energy into the Eu(3+) energy level, was an energetically enhanced europium emission. Typical stacking behaviors of π-π interactions between the ligands and the Eu(3+)-ion were analyzed using single crystal X-ray diffraction. Regarding the luminescence performance of this europium composite, the ligand/defect emission was suppressed by dispersion in a poly-BMA (PBMA) matrix. The underlying mechanism of the effective enhancement of the pure Eu(3+) emission was attributed to the combined effects of structural modifications, defect emissions, and carrier charge transfer. Fluorescence spectra were compared to the composite of optimized Eu3+ emission where they were subsequently chelated to four metal ions via carboxylate groups on the BMA unit. The optical enhanced europium composite clearly demonstrated highly efficient optical responses and is, therefore a promising application as an optical detection material.
Keywords: UV-curing; europium complex; fluorescence detection; metal-ion chelating; optical tuning.
Figures






Similar articles
-
Luminescence of Eu(DBM)3Phen-doped in azobenzene-containing copolymers--effects of absorption overlapping of two components.Spectrochim Acta A Mol Biomol Spectrosc. 2010 Mar;75(3):992-6. doi: 10.1016/j.saa.2009.12.024. Epub 2009 Dec 16. Spectrochim Acta A Mol Biomol Spectrosc. 2010. PMID: 20060776
-
Fluorescence enhancement of europium (III) perchlorate by 1,10-phenanthroline on the 1-(naphthalen-2-yl)-2-(phenylsulthio)ethanone complex and luminescence mechanism.Luminescence. 2014 Nov;29(7):810-7. doi: 10.1002/bio.2625. Epub 2014 Jan 16. Luminescence. 2014. PMID: 24436058
-
Luminescent europium and terbium complexes of dipyridoquinoxaline and dipyridophenazine ligands as photosensitizing antennae: structures and biological perspectives.Dalton Trans. 2015 Dec 14;44(46):19844-55. doi: 10.1039/c5dt02852c. Dalton Trans. 2015. PMID: 26507987
-
Unveiling the Versatility of Coumarin-Integrated with Phenanthroline/Thiabendazole-Based EuIII Complexes for Smart LEDs, Vapoluminescence, and Bioimaging Applications.ACS Appl Bio Mater. 2024 Sep 16;7(9):5795-5809. doi: 10.1021/acsabm.4c00839. Epub 2024 Aug 12. ACS Appl Bio Mater. 2024. PMID: 39279416 Review.
-
Europium(II/III) coordination chemistry toward applications.Chem Commun (Camb). 2024 Sep 24;60(77):10655-10671. doi: 10.1039/d4cc03080j. Chem Commun (Camb). 2024. PMID: 39230388 Free PMC article. Review.
Cited by
-
Highly Sensitive and Selective Fluorescence "Turn-On" Detection of Pb (II) Based on Fe3O4@Au-FITC Nanocomposite.Molecules. 2021 May 26;26(11):3180. doi: 10.3390/molecules26113180. Molecules. 2021. PMID: 34073353 Free PMC article.
References
-
- Lenaerts P., Driesen K., Van Deun R., Binnemans K. Covalent coupling of luminescent tris(2-thenoyl-trifluoroacetonato)lanthanide(III) complexes on a merrifield resin. Chem. Mater. 2005;17:2148–2154.
-
- Bergstedt M.D.T., Zhang C., Saab A.P., O’Regan M.B, Bazan G.C., Srdanov V.I., Heeger A.J. Narrow bandwidth luminescence from blends with energy transfer from semiconducting conjugated polymers to europium complexes. Adv. Mater. 1999;11:1349–1354.
-
- Hong Z.R., Liang C.J., Li R.G., Li W.L., Zhao D., Fan D., Wang D.Y., Chu B., Zang F.X., Hong L.S., Lee S.T. Rare earth complex as a high-efficiency emitter in an electroluminescent device. Adv. Mater. 2001;13:1241–1245.
-
- Shunmugam R., Tew G.N. Polymers that contain ligand metals in their side chain: building foundation for functional materials in optoelectronic applications with an emphasis on lanthanide ions. Macromol. Rapid Commun. 2008;29:1355–1362.
-
- Xin H., Li F.Y., Guan M., Huang C.H., Sun M., Wang K.Z., Zhang Y.A., Jin L.P. Carbazole-functionalized europium complex and its high-efficiency organic electroluminescent properties. J. Appl. Phys. 2003;94:4729–4731.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

