Inroads to predict in vivo toxicology-an introduction to the eTOX Project
- PMID: 22489185
- PMCID: PMC3317745
- DOI: 10.3390/ijms13033820
Inroads to predict in vivo toxicology-an introduction to the eTOX Project
Abstract
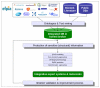
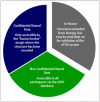
There is a widespread awareness that the wealth of preclinical toxicity data that the pharmaceutical industry has generated in recent decades is not exploited as efficiently as it could be. Enhanced data availability for compound comparison ("read-across"), or for data mining to build predictive tools, should lead to a more efficient drug development process and contribute to the reduction of animal use (3Rs principle). In order to achieve these goals, a consortium approach, grouping numbers of relevant partners, is required. The eTOX ("electronic toxicity") consortium represents such a project and is a public-private partnership within the framework of the European Innovative Medicines Initiative (IMI). The project aims at the development of in silico prediction systems for organ and in vivo toxicity. The backbone of the project will be a database consisting of preclinical toxicity data for drug compounds or candidates extracted from previously unpublished, legacy reports from thirteen European and European operation-based pharmaceutical companies. The database will be enhanced by incorporation of publically available, high quality toxicology data. Seven academic institutes and five small-to-medium size enterprises (SMEs) contribute with their expertise in data gathering, database curation, data mining, chemoinformatics and predictive systems development. The outcome of the project will be a predictive system contributing to early potential hazard identification and risk assessment during the drug development process. The concept and strategy of the eTOX project is described here, together with current achievements and future deliverables.
Keywords: Data Integration; Decision Support System; Expert Systems; Knowledge Management; Manual Curation; QSAR; computational models; data sharing; histopathology; in silico toxicity; in vitro toxicity; in vivo toxicity; ontology; predictive toxicology.
Figures




References
-
- ICH Topic M 3 (R2): Non-Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals CPMP/ICH/286/95. [accessed on 19 September 2011]. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin....
-
- Federsel H.J. Handing Over the Baton: Connecting Medicinal Chemistry with Process R&D. Drug News Perspect. 2008;21:193–199. - PubMed
-
- Car B. Enabling Technologies in Reducing Drug Attrition Due to Safety Failures. Int. Drug Disc. 2006;1:53–56.
-
- Morelli J.K., Buehrle M., Pognan F., Barone L., Fieles W., Ciaccio P.J. Validation of an in vitro screen for phopholipidosis using a high content biology platform. Cell Biol. Toxicol. 2006;22:15–27. - PubMed
-
- Hancox J.C., McPate M.J., El Harchi A., Zhang Y.H. The hERG potassium channel and hERG screening for drug-induced Torsades de Pointes. Pharmacol. Ther. 2008;119:118–132. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

