Peroxiredoxin III and sulfiredoxin together protect mice from pyrazole-induced oxidative liver injury
- PMID: 22490042
- PMCID: PMC3437045
- DOI: 10.1089/ars.2011.4334
Peroxiredoxin III and sulfiredoxin together protect mice from pyrazole-induced oxidative liver injury
Abstract
Aims: To define the mechanisms underlying pyrazole-induced oxidative stress and the protective role of peroxiredoxins (Prxs) and sulfiredoxin (Srx) against such stress.
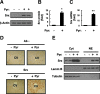
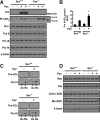
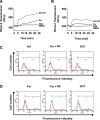
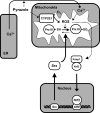
Results: Pyrazole increased Srx expression in the liver of mice in a nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent manner and induced Srx translocation from the cytosol to the endoplasmic reticulum (ER) and mitochondria. Pyrazole also induced the expression of CYP2E1, a primary reactive oxygen species (ROS) source for ethanol-induced liver injury, in ER and mitochondria. However, increased CYP2E1 levels only partially accounted for the pyrazole-mediated induction of Srx, prompting the investigation of CYP2E1-independent ROS generation downstream of pyrazole. Indeed, pyrazole increased ER stress, which is known to elevate mitochondrial ROS. In addition, pyrazole up-regulated CYP2E1 to a greater extent in mitochondria than in ER. Accordingly, among Prxs I to IV, PrxIII, which is localized to mitochondria, was preferentially hyperoxidized in the liver of pyrazole-treated mice. Pyrazole-induced oxidative damage to the liver was greater in PrxIII(-/-) mice than in wild-type mice. Such damage was also increased in Srx(-/-) mice treated with pyrazole, underscoring the role of Srx as the guardian of PrxIII.
Innovation: The roles of Prxs, Srx, and ER stress have not been previously studied in relation to pyrazole toxicity.
Conclusion: The concerted action of PrxIII and Srx is important for protection against pyrazole-induced oxidative stress arising from the convergent induction of CYP2E1-derived and ER stress-derived ROS in mitochondria.
Figures




References
-
- Anandatheerthavarada HK. Addya S. Dwivedi RS. Biswas G. Mullick J. Avadhani NG. Localization of multiple forms of inducible cytochromes P450 in rat liver mitochondria: immunological characteristics and patterns of xenobiotic substrate metabolism. Arch Biochem Biophys. 1997;339:136–150. - PubMed
-
- Bae SH. Sung SH. Cho EJ. Lee SK. Lee HE. Woo HA. Yu D. Kil IS. Rhee SG. Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver. Hepatology. 2011;53:945–953. - PubMed
-
- Bae SH. Woo HA. Sung SH. Lee HE. Lee SK. Kil IS. Rhee SG. Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia. Antioxid Redox Signal. 2009;11:937–948. - PubMed
-
- Bai J. Cederbaum AI. Overexpression of CYP2E1 in mitochondria sensitizes HepG2 cells to the toxicity caused by depletion of glutathione. J Biol Chem. 2006;281:5128–5136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

