Rheological and biological properties of a hydrogel support for cells intended for intervertebral disc repair
- PMID: 22490206
- PMCID: PMC3375205
- DOI: 10.1186/1471-2474-13-54
Rheological and biological properties of a hydrogel support for cells intended for intervertebral disc repair
Abstract
Background: Cell-based approaches towards restoration of prolapsed or degenerated intervertebral discs are hampered by a lack of measures for safe administration and placement of cell suspensions within a treated disc. In order to overcome these risks, a serum albumin-based hydrogel has been developed that polymerizes after injection and anchors the administered cell suspension within the tissue.
Methods: A hydrogel composed of chemically activated albumin crosslinked by polyethylene glycol spacers was produced. The visco-elastic gel properties were determined by rheological measurement. Human intervertebral disc cells were cultured in vitro and in vivo in the hydrogel and their phenotype was tested by reverse-transcriptase polymerase chain reaction. Matrix production and deposition was monitored by immuno-histology and by biochemical analysis of collagen and glycosaminoglycan deposition. Species specific in situ hybridization was performed to discriminate between cells of human and murine origin in xenotransplants.
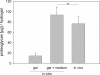
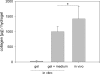
Results: The reproducibility of the gel formation process could be demonstrated. The visco-elastic properties were not influenced by storage of gel components. In vitro and in vivo (subcutaneous implants in mice) evidence is presented for cellular differentiation and matrix deposition within the hydrogel for human intervertebral disc cells even for donor cells that have been expanded in primary monolayer culture, stored in liquid nitrogen and re-activated in secondary monolayer culture. Upon injection into the animals, gels formed spheres that lasted for the duration of the experiments (14 days). The expression of cartilage- and disc-specific mRNAs was maintained in hydrogels in vitro and in vivo, demonstrating the maintenance of a stable specific cellular phenotype, compared to monolayer cells. Significantly higher levels of hyaluronan synthase isozymes-2 and -3 mRNA suggest cell functionalities towards those needed for the support of the regeneration of the intervertebral disc. Moreover, mouse implanted hydrogels accumulated 5 times more glycosaminoglycans and 50 times more collagen than the in vitro cultured gels, the latter instead releasing equivalent quantities of glycosaminoglycans and collagen into the culture medium. Matrix deposition could be specified by immunohistology for collagen types I and II, and aggrecan and was found only in areas where predominantly cells of human origin were detected by species specific in situ hybridization.
Conclusions: The data demonstrate that the hydrogels form stable implants capable to contain a specifically functional cell population within a physiological environment.
Figures




References
-
- Scholz B, Kinzelmann C, Benz K, Mollenhauer J, Wurst H, Schlosshauer B. Suppression of adverse angiogenesis in an albumin-based hydrogel for articular cartilage and intervertebral disc regeneration. Eur Cell Mater. 2010;20:24–36. discussion 36-27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

