Comparison of cerebrospinal fluid levels of tau and Aβ 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms
- PMID: 22490326
- PMCID: PMC3528180
- DOI: 10.1001/archneurol.2012.26
Comparison of cerebrospinal fluid levels of tau and Aβ 1-42 in Alzheimer disease and frontotemporal degeneration using 2 analytical platforms
Abstract
Objective: To use values of cerebrospinal fluid tau and β-amyloid obtained from 2 different analytical immunoassays to differentiate Alzheimer disease (AD) from frontotemporal lobar degeneration (FTLD).
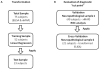
Design: Cerebrospinal fluid values of total tau (T-tau) and β-amyloid 1-42 (Aβ 1-42) obtained using the Innotest enzyme-linked immunosorbent assay were transformed using a linear regression model to equivalent values obtained using the INNO-BIA AlzBio3 (xMAP; Luminex) assay. Cutoff values obtained from the xMAP assay were developed in a series of autopsy-confirmed cases and cross validated in another series of autopsy-confirmed samples using transformed enzyme-linked immunosorbent assay values to assess sensitivity and specificity for differentiating AD from FTLD.
Setting: Tertiary memory disorder clinics and neuropathologic and biomarker core centers.
Participants: Seventy-five samples from patients with cerebrospinal fluid data obtained from both assays were used for transformation of enzyme-linked immunosorbent assay values. Forty autopsy-confirmed cases (30 with AD and 10 with FTLD) were used to establish diagnostic cutoff values and then cross validated in a second sample set of 21 autopsy-confirmed cases (11 with AD and 10 with FTLD) with transformed enzyme-linked immunosorbent assay values.
Main outcome measure: Diagnostic accuracy using transformed biomarker values.
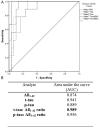
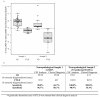
Results: Data obtained from both assays were highly correlated. The T-tau to Aβ 1-42 ratio had the highest correlation between measures (r = 0.928, P < .001) and high reliability of transformation (intraclass correlation coefficient= 0.89). A cutoff of 0.34 for the T-tau to Aβ 1-42 ratio had 90% and 100% sensitivity and 96.7% and 91% specificity to differentiate FTLD cases in the validation and cross-validation samples, respectively.
Conclusions: Values from 2 analytical platforms can be transformed into equivalent units, which can distinguish AD from FTLD more accurately than the clinical diagnosis.
Figures

References
-
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999 Oct;56(10):1233–1239. - PubMed
-
- Grossman M, Libon DJ, Forman MS, et al. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Arch Neurol. 2007 Nov;64(11):1601–1609. - PubMed
-
- Boeve BF, Maraganore DM, Parisi JE, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999 Sep 11;53(4):795–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG010124/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- P01 AG32953/AG/NIA NIH HHS/United States
- P50 NS053488/NS/NINDS NIH HHS/United States
- P01 NS53488/NS/NINDS NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- P30AG010124-20/AG/NIA NIH HHS/United States
- R01 NS044266/NS/NINDS NIH HHS/United States
- P01 AG032953/AG/NIA NIH HHS/United States
- R01 AG15116/AG/NIA NIH HHS/United States
- R01 AG015116/AG/NIA NIH HHS/United States
- R01 NS44266/NS/NINDS NIH HHS/United States
- T32 AG000255/AG/NIA NIH HHS/United States
- T32-AG000255/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

