M2 muscarinic acetylcholine receptors regulate long-term potentiation at hippocampal CA3 pyramidal cell synapses in an input-specific fashion
- PMID: 22490561
- PMCID: PMC3434620
- DOI: 10.1152/jn.00740.2011
M2 muscarinic acetylcholine receptors regulate long-term potentiation at hippocampal CA3 pyramidal cell synapses in an input-specific fashion
Abstract
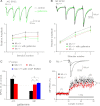
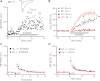
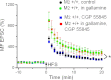
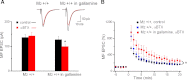
Muscarinic receptors have long been known as crucial players in hippocampus-dependent learning and memory, but our understanding of the cellular underpinnings and the receptor subtypes involved lags well behind. This holds in particular for the hippocampal CA3 region, where the mechanisms of synaptic plasticity depend on the type of afferent input. Williams and Johnston (Williams S, Johnston D. Science 242: 84-87, 1988; Williams S, Johnston D. J Neurophysiol 64: 1089-1097, 1990) demonstrated muscarinic depression of mossy fiber (MF) long-term potentiation (LTP) through a presynaptic site of action and Maeda et al. (Maeda T, Kaneko S, Satoh M. Brain Res 619: 324-330, 1993) proposed a bidirectional modulation of MF LTP by muscarinic receptor subtypes. Since then, this issue, as well as muscarinic regulation of plasticity at associational/commissural (A/C) fiber-CA3 synapses has remained largely neglected, not least because of the lack of highly selective ligands for the different muscarinic receptor subtypes. In the present study, we performed field potential and whole cell recordings from the hippocampal CA3 region of M(2) receptor knockout mice to determine the role of M(2) receptors in short-term and long-term plasticity at A/C and MF inputs to CA3 pyramidal cells. At the A/C synapse, M(2) receptors promoted short-term facilitation and LTP. Unexpectedly, M(2) receptors mediated the opposite effect on LTP at the MF synapse, which was significantly reduced, most likely involving a depressant effect of M(2) receptors on adenylyl cyclase activity in MF terminals. Our data demonstrate that cholinergic projections recruit M(2) receptors to redistribute the gain of LTP in CA3 pyramidal cells in an input-specific manner.
Figures


References
-
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6: 51–58, 2003 - PubMed
-
- Auerbach JM, Segal M. A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol 72: 2034–2040, 1994 - PubMed
-
- Bancila V, Cordeiro JM, Bloc A, Dunant Y. Nicotine-induced and depolarisation-induced glutamate release from hippocampus mossy fibre synaptosomes: two distinct mechanisms. J Neurochem 110: 570–580, 2009 - PubMed
-
- Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci 41: 340–346, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

