Maturing reticulocytes internalize plasma membrane in glycophorin A-containing vesicles that fuse with autophagosomes before exocytosis
- PMID: 22490681
- PMCID: PMC3383192
- DOI: 10.1182/blood-2011-09-376475
Maturing reticulocytes internalize plasma membrane in glycophorin A-containing vesicles that fuse with autophagosomes before exocytosis
Abstract
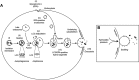
The erythrocyte is one of the best characterized human cells. However, studies of the process whereby human reticulocytes mature to erythrocytes have been hampered by the difficulty of obtaining sufficient numbers of cells for analysis. In the present study, we describe an in vitro culture system producing milliliter quantities of functional mature human adult reticulocytes from peripheral blood CD34(+) cells. We show that the final stage of reticulocyte maturation occurs by a previously undescribed mechanism in which large glycophorin A-containing vesicles forming at the cytosolic face of the plasma membrane are internalized and fuse with autophagosomes before expulsion of the autophagosomal contents by exocytosis. Early reticulocyte maturation is characterized by the selective elimination of unwanted plasma membrane proteins (CD71, CD98, and β1 integrin) through the endosome-exosome pathway. In contrast, late maturation is characterized by the generation of large glycophorin A-decorated vesicles of autophagic origin.
Figures
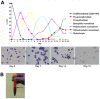

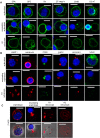
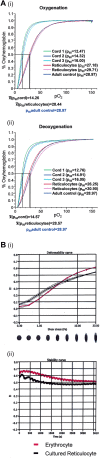

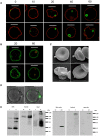
Comment in
-
The ins and outs of human reticulocyte maturation: autophagy and the endosome/exosome pathway.Autophagy. 2012 Jul 1;8(7):1150-1. doi: 10.4161/auto.20648. Epub 2012 Jun 4. Autophagy. 2012. PMID: 22659916 Free PMC article.
References
-
- Mel HC, Prenant M, Mohandas N. Reticulocyte motility and form: studies on maturation and classification. Blood. 1977;49(6):1001–1009. - PubMed
-
- Chasis JA, Prenant M, Leung A, Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989;74(3):1112–1120. - PubMed
-
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

