A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers
- PMID: 22491086
- PMCID: PMC3390009
- DOI: 10.1093/aje/kwr401
A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers
Abstract
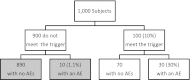
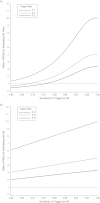
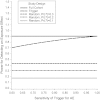
The Pediatric HIV/AIDS Cohort Study's Surveillance Monitoring of ART Toxicities Study is a prospective cohort study conducted at 22 US sites between 2007 and 2011 that was designed to evaluate the safety of in utero antiretroviral drug exposure in children not infected with human immunodeficiency virus who were born to mothers who were infected. This ongoing study uses a "trigger-based" design; that is, initial assessments are conducted on all children, and only those meeting certain thresholds or "triggers" undergo more intensive evaluations to determine whether they have had an adverse event (AE). The authors present the estimated rates of AEs for each domain of interest in the Surveillance Monitoring of ART Toxicities Study. They also evaluated the efficiency of this trigger-based design for estimating AE rates and for testing associations between in utero exposures to antiretroviral drugs and AEs. The authors demonstrate that estimated AE rates from the trigger-based design are unbiased after correction for the sensitivity of the trigger for identifying AEs. Even without correcting for bias based on trigger sensitivity, the trigger approach is generally more efficient for estimating AE rates than is evaluating a random sample of the same size. Minor losses in efficiency when comparing AE rates between persons exposed and unexposed in utero to particular antiretroviral drugs or drug classes were observed under most scenarios.
Figures
References
-
- Townsend CL, Cortina-Borja M, Peckham CS, et al. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22(8):973–981. - PubMed
-
- Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. Women and Infants’ Transmission Study Group. J Acquir Immune Defic Syndr. 2002;29(5):484–494. - PubMed
-
- Suksomboon N, Poolsup N, Ket-aim S. Systematic review of the efficacy of antiretroviral therapies for reducing the risk of mother-to-child transmission of HIV infection. J Clin Pharm Ther. 2007;32(3):293–311. - PubMed
-
- Public Health Service recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Morb Mortal Wkly Rep. 1998;47(RR-2):1–38. - PubMed
-
- European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. 2003;32(4):380–387. - PubMed

