Staged decline of neuronal function in vivo in an animal model of Alzheimer's disease
- PMID: 22491322
- PMCID: PMC3337977
- DOI: 10.1038/ncomms1783
Staged decline of neuronal function in vivo in an animal model of Alzheimer's disease
Abstract
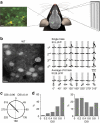
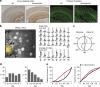
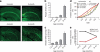
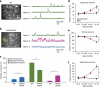
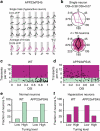
The accumulation of amyloid-β in the brain is an essential feature of Alzheimer's disease. However, the impact of amyloid-β-accumulation on neuronal dysfunction on the single cell level in vivo is poorly understood. Here we investigate the progression of amyloid-β load in relation to neuronal dysfunction in the visual system of the APP23×PS45 mouse model of Alzheimer's disease. Using in vivo two-photon calcium imaging in the visual cortex, we demonstrate that a progressive deterioration of neuronal tuning for the orientation of visual stimuli occurs in parallel with the age-dependent increase of the amyloid-β load. Importantly, we find this deterioration only in neurons that are hyperactive during spontaneous activity. This impairment of visual cortical circuit function also correlates with pronounced deficits in visual-pattern discrimination. Together, our results identify distinct stages of decline in sensory cortical performance in vivo as a function of the increased amyloid-β-load.
Figures

References
-
- Blennow K., de Leon M. J. & Zetterberg H. Alzheimer's disease. Lancet 368, 387–403 (2006). - PubMed
-
- Nestor P. J., Scheltens P. & Hodges J. R. Advances in the early detection of Alzheimer's disease. Nat. Med. 10(Suppl), S34–S41 (2004). - PubMed
-
- Djordjevic J., Jones-Gotman M., De Sousa K. & Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging 29, 693–706 (2008). - PubMed
-
- Mesholam R. I., Moberg P. J., Mahr R. N. & Doty R. L. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch. Neurol. 55, 84–90 (1998). - PubMed
-
- Devanand D. P. et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow-up. Am. J. Psychiatry 157, 1399–1405 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

