Propranolol inhibition of β-adrenergic receptor does not suppress pathologic neovascularization in oxygen-induced retinopathy
- PMID: 22491401
- PMCID: PMC3376075
- DOI: 10.1167/iovs.12-9691
Propranolol inhibition of β-adrenergic receptor does not suppress pathologic neovascularization in oxygen-induced retinopathy
Abstract
Purpose: Retinopathy of prematurity (ROP) is a leading cause of blindness in children and is, in its most severe form, characterized by uncontrolled growth of vision-threatening pathologic vessels. Propranolol, a nonselective β-adrenergic receptor blocker, was reported to protect against pathologic retinal neovascularization in a mouse model of oxygen-induced retinopathy (OIR). Based on this single animal study using nonstandard evaluation of retinopathy, clinical trials are currently ongoing to evaluate propranolol treatment in stage 2 ROP patients who tend to experience spontaneous disease regression and are at low risk of blindness. Because these ROP patients are vulnerable premature infants who are still in a fragile state of incomplete development, the efficacy of propranolol treatment in retinopathy needs to be evaluated thoroughly in preclinical animal models of retinopathy and potential benefits weighed against potential adverse effects.
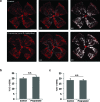
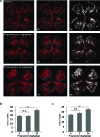
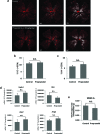
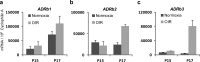
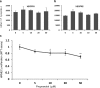
Methods: Retinopathy was induced by exposing neonatal mice to 75% oxygen from postnatal day (P) 7 to P12. Three routes of propranolol treatment were assessed from P12 to P16: oral gavage, intraperitoneal injection, or subcutaneous injection, with doses varying between 2 and 60 mg/kg/day. At P17, retinal flatmounts were stained with isolectin and quantified with a standard protocol to measure vasoobliteration and pathologic neovascularization. Retinal gene expression was analyzed with qRT-PCR using RNA isolated from retinas of control and propranolol-treated pups.
Results: None of the treatment approaches at any dose of propranolol (up to 60 mg/kg/day) were effective in preventing the development of retinopathy in a mouse model of OIR, evaluated using standard techniques. Propranolol treatment also did not change retinal expression of angiogenic factors including vascular endothelial growth factor.
Conclusions: Propranolol treatment via three routes and up to 30 times the standard human dose failed to suppress retinopathy development in mice. These data bring into question whether propranolol through inhibition of β-adrenergic receptors is an appropriate therapeutic approach for treating ROP.
Conflict of interest statement
Disclosure:
Figures

Comment in
-
Different efficacy of propranolol in mice with oxygen-induced retinopathy: could differential effects of propranolol be related to differences in mouse strains?Invest Ophthalmol Vis Sci. 2012 Oct 30;53(11):7421-3. doi: 10.1167/iovs.12-10721. Invest Ophthalmol Vis Sci. 2012. PMID: 23111729 No abstract available.
References
-
- Chen J, Smith LEH. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140 - PubMed
-
- Léauté-Labrèze Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651 - PubMed
-
- Price CJ, Lattouf C, Baum B, et al. Propranolol vs corticosteroids for infantile hemangiomas: a multicenter retrospective analysis. Arch Dermatol. 2011;147:1371–1376 - PubMed
-
- Manunza F, Syed S, Laguda B, et al. Propranolol for complicated infantile haemangiomas: a case series of 30 infants. Br J Dermatol. 2010;162:466–468 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

