Phosphorylation of phosducin accelerates rod recovery from transducin translocation
- PMID: 22491418
- PMCID: PMC3382380
- DOI: 10.1167/iovs.11-8798
Phosphorylation of phosducin accelerates rod recovery from transducin translocation
Abstract
Purpose: In rods saturated by light, the G protein transducin undergoes translocation from the outer segment compartment, which results in the uncoupling of transducin from its innate receptor, rhodopsin. We measured the kinetics of recovery from this adaptive cellular response, while also investigating the role of phosducin, a phosphoprotein binding transducin βγ subunits in its de-phosphorylated state, in regulating this process.
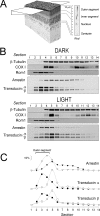
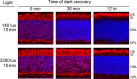
Methods: Mice were exposed to a moderate rod-saturating light triggering transducin translocation, and then allowed to recover in the dark while free running. The kinetics of the return of the transducin subunits to the outer segments were compared in transgenic mouse models expressing full-length phosducin, and phosducin lacking phosphorylation sites serine 54 and 71, using Western blot analysis of serial tangential sections of the retina.
Results: In mice expressing normal phosducin, transducin α and βγ subunits returned to the outer segments with a half-time (t(1/2)) of ∼24 and 29 minutes, respectively. In the phosducin phosphorylation mutants, the transducin α subunit moved four times slower, with t(1/2) ∼95 minutes, while the movement of transducin βγ was less affected.
Conclusions: We demonstrate that the recovery of rod photoreceptors from the ambient saturating levels of illumination, in terms of the return of the light-dispersed transducin subunits to the rod outer segments, occurs six times faster than reported previously. Our data also support the notion that the accumulation of transducin α subunit in the outer segment is driven by its re-binding to the transducin βγ dimer, because this process is accelerated significantly by phosducin phosphorylation.
Conflict of interest statement
Disclosure:
Figures

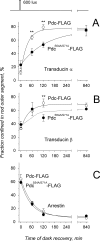
 . The half-time (
. The half-time (
 ) of transducin α return to the rod outer segment was 24 and 95 minutes in Pdc-FLAG mice and in PdcS54A/S71A-FLAG mice, respectively. (B) Similar analysis as in (A) revealed t1/2 = 29 minutes for the transducin β movement in Pdc-FLAG mice. Movement of this subunit in the PdcS54A/S71A-FLAG strain did not obey exponential kinetics (dashed line) and its t1/2 could not be determined. (C) Fitting of the arrestin data from Table 1 was performed using an exponential decay function
) of transducin α return to the rod outer segment was 24 and 95 minutes in Pdc-FLAG mice and in PdcS54A/S71A-FLAG mice, respectively. (B) Similar analysis as in (A) revealed t1/2 = 29 minutes for the transducin β movement in Pdc-FLAG mice. Movement of this subunit in the PdcS54A/S71A-FLAG strain did not obey exponential kinetics (dashed line) and its t1/2 could not be determined. (C) Fitting of the arrestin data from Table 1 was performed using an exponential decay function
 . Arrestin moves from the outer segment with t1/2 = 44 and 38 minutes in Pdc-FLAG and PdcS54A/S71A-FLAG mice, respectively. For all data a t-test was applied: no asterisk indicates a P value > 0.1; (**) indicates a P value < 0.05.
. Arrestin moves from the outer segment with t1/2 = 44 and 38 minutes in Pdc-FLAG and PdcS54A/S71A-FLAG mice, respectively. For all data a t-test was applied: no asterisk indicates a P value > 0.1; (**) indicates a P value < 0.05.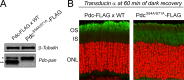
References
-
- Lee RH , Lieberman BS , Lolley RN . A novel complex from bovine visual cells of a 33,000-dalton phosphoprotein with beta- and gamma-transducin: purification and subunit structure. Biochemistry. 1987;26:3983–3990 - PubMed
-
- Gaudet R , Bohm A , Sigler PB . Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell. 1996;87:577–588 - PubMed
-
- Hawes BE , Touhara K , Kurose H , Lefkowitz RJ , Inglese J . Determination of the G beta gamma-binding domain of phosducin. A regulatable modulator of G beta gamma signaling. J Biol Chem. 1994;269:29825–29830 - PubMed
-
- Muller S , Straub A , Schröder S , Bauer PH , Lohse MJ . Interactions of phosducin with defined G protein beta gamma-subunits. J Biol Chem. 1996;271:11781–11786 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

