A 3-O-sulfated heparan sulfate binding peptide preferentially targets herpes simplex virus 2-infected cells
- PMID: 22491462
- PMCID: PMC3393556
- DOI: 10.1128/JVI.00433-12
A 3-O-sulfated heparan sulfate binding peptide preferentially targets herpes simplex virus 2-infected cells
Abstract
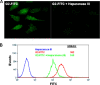
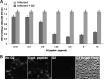
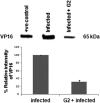
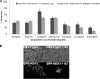
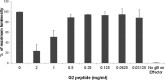
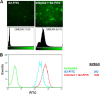
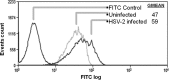
Herpes simplex virus 2 (HSV-2) is the primary cause of genital herpes, which is one of the most common sexually transmitted viral infections worldwide and a major cofactor for human immunodeficiency virus infection. The lack of an effective vaccine or treatment and the emergence of drug-resistant strains highlight the need for developing new antivirals for HSV-2. Here, we demonstrate that a low-molecular-weight peptide isolated against 3-O-sulfated heparan sulfate (3-OS HS) can efficiently block HSV-2 infection. Treatment with the peptide inhibited viral entry and cell-to-cell spread both in vitro and in vivo using a mouse model of genital HSV-2 infection. Quite interestingly, the peptide showed a preferential binding to HSV-2-infected cells, with more than 200% increased binding compared to uninfected cells. Our additional results show that heparan sulfate expression is upregulated by 25% upon HSV-2 infection, which is a significant new finding that could be exploited for designing new diagnostic tests and treatment strategies against HSV-2-infected cells. In addition, our results also raise the possibility that 3-OS HS modifications within HS may be upregulated even more to accommodate for a significantly higher increase in the peptide binding to the infected cells.
Figures


References
-
- Albiol Matanic VC, Castilla V. 2004. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents 23:382–389 - PubMed
-
- Andersen JH, Jenssen H, Gutteberg TJ. 2003. Lactoferrin and lactoferricin inhibit herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antiviral Res. 58:209–215 - PubMed
-
- Andersen JH, Jenssen H, Sandvik K, Gutteberg TJ. 2004. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 74:262–271 - PubMed
-
- Araki-Sasaki K, et al. 1995. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest. Ophthalmol. Vis. Sci. 36:614–621 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

