Oct4+ stem/progenitor swine lung epithelial cells are targets for influenza virus replication
- PMID: 22491467
- PMCID: PMC3393566
- DOI: 10.1128/JVI.00341-12
Oct4+ stem/progenitor swine lung epithelial cells are targets for influenza virus replication
Abstract
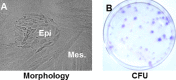
We isolated stem/progenitor epithelial cells from the lungs of 4- to 6-week-old pigs. The epithelial progenitor colony cells were surrounded by mesenchymal stromal cells. The progenitor epithelial colony cells expressed stem cell markers such as octamer binding transcription factor 4 (Oct4) and stage-specific embryonic antigen 1 (SSEA-1), as well as the epithelial markers pancytokeratin, cytokeratin-18, and occludin, but not mesenchymal (CD44, CD29, and CD90) and hematopoietic (CD45) markers. The colony cells had extensive self-renewal potential and had the capacity to undergo differentiation to alveolar type I- and type II-like pneumocytes. Additionally, these cells expressed sialic acid receptors and supported the active replication of influenza virus, which was accompanied by cell lysis. The lysis of progenitor epithelial cells by influenza virus may cause a marked reduction in the potential of progenitor cells for self renewal and for their ability to differentiate into specialized cells of the lung. These observations suggest the possible involvement of lung stem/progenitor cells in influenza virus infection.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

