Synapses and Alzheimer's disease
- PMID: 22491782
- PMCID: PMC3331702
- DOI: 10.1101/cshperspect.a005777
Synapses and Alzheimer's disease
Abstract
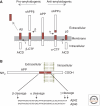
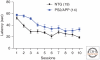
Alzheimer's disease (AD) is a major cause of dementia in the elderly. Pathologically, AD is characterized by the accumulation of insoluble aggregates of Aβ-peptides that are proteolytic cleavage products of the amyloid-β precursor protein ("plaques") and by insoluble filaments composed of hyperphosphorylated tau protein ("tangles"). Familial forms of AD often display increased production of Aβ peptides and/or altered activity of presenilins, the catalytic subunits of γ-secretase that produce Aβ peptides. Although the pathogenesis of AD remains unclear, recent studies have highlighted two major themes that are likely important. First, oligomeric Aβ species have strong detrimental effects on synapse function and structure, particularly on the postsynaptic side. Second, decreased presenilin function impairs synaptic transmission and promotes neurodegeneration. The mechanisms underlying these processes are beginning to be elucidated, and, although their relevance to AD remains debated, understanding these processes will likely allow new therapeutic avenues to AD.
Figures


References
-
- Ballatore C, Lee VM, Trojanowski JQ 2007. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 8: 663–672 - PubMed
-
- Bancher C, Braak H, Fischer P, Jellinger KA 1993. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer’s and Parkinson’s disease patients. Neurosci Lett 162: 179–182 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
