Effects of fetal antiepileptic drug exposure: outcomes at age 4.5 years
- PMID: 22491865
- PMCID: PMC3324322
- DOI: 10.1212/WNL.0b013e318250d824
Effects of fetal antiepileptic drug exposure: outcomes at age 4.5 years
Erratum in
- Neurology. 2012 May 15;78(20):1622
Abstract
Objective: To examine outcomes at age 4.5 years and compare to earlier ages in children with fetal antiepileptic drug (AED) exposure.
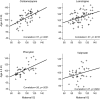
Methods: The NEAD Study is an ongoing prospective observational multicenter study, which enrolled pregnant women with epilepsy on AED monotherapy (1999-2004) to determine if differential long-term neurodevelopmental effects exist across 4 commonly used AEDs (carbamazepine, lamotrigine, phenytoin, or valproate). The primary outcome is IQ at 6 years of age. Planned analyses were conducted using Bayley Scales of Infant Development (BSID at age 2) and Differential Ability Scale (IQ at ages 3 and 4.5).
Results: Multivariate intent-to-treat (n = 310) and completer (n = 209) analyses of age 4.5 IQ revealed significant effects for AED group. IQ for children exposed to valproate was lower than each other AED. Adjusted means (95% confidence intervals) were carbamazepine 106 (102-109), lamotrigine 106 (102-109), phenytoin 105 (102-109), valproate 96 (91-100). IQ was negatively associated with valproate dose, but not other AEDs. Maternal IQ correlated with child IQ for children exposed to the other AEDs, but not valproate. Age 4.5 IQ correlated with age 2 BSID and age 3 IQ. Frequency of marked intellectual impairment diminished with age except for valproate (10% with IQ <70 at 4.5 years). Verbal abilities were impaired for all 4 AED groups compared to nonverbal skills.
Conclusions: Adverse cognitive effects of fetal valproate exposure persist to 4.5 years and are related to performances at earlier ages. Verbal abilities may be impaired by commonly used AEDs. Additional research is needed.
Figures
References
-
- Fisher JE, Vorhees CV. Developmental toxicity of antiepileptic drugs: relationship to postnatal dysfunction. Pharmacol Res 1992;26:207–221 - PubMed
-
- Gaily E, Meador KJ. Neurodevelopmental effects. In: Engel J, Pedley TA. eds. Epilepsy: A Comprehensive Textbook, 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2007: 1225–1233
-
- FDA Drug Safety Communication. Children born to mothers who took Valproate products while pregnant may have impaired cognitive development (6/30/2011). [Accessed March 1, 2012.]. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm261543.htm.