Mitotic spindle form and function
- PMID: 22491889
- PMCID: PMC3316638
- DOI: 10.1534/genetics.111.128710
Mitotic spindle form and function
Abstract
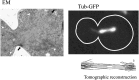
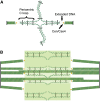
The Saccharomyces cerevisiae mitotic spindle in budding yeast is exemplified by its simplicity and elegance. Microtubules are nucleated from a crystalline array of proteins organized in the nuclear envelope, known as the spindle pole body in yeast (analogous to the centrosome in larger eukaryotes). The spindle has two classes of nuclear microtubules: kinetochore microtubules and interpolar microtubules. One kinetochore microtubule attaches to a single centromere on each chromosome, while approximately four interpolar microtubules emanate from each pole and interdigitate with interpolar microtubules from the opposite spindle to provide stability to the bipolar spindle. On the cytoplasmic face, two to three microtubules extend from the spindle pole toward the cell cortex. Processes requiring microtubule function are limited to spindles in mitosis and to spindle orientation and nuclear positioning in the cytoplasm. Microtubule function is regulated in large part via products of the 6 kinesin gene family and the 1 cytoplasmic dynein gene. A single bipolar kinesin (Cin8, class Kin-5), together with a depolymerase (Kip3, class Kin-8) or minus-end-directed kinesin (Kar3, class Kin-14), can support spindle function and cell viability. The remarkable feature of yeast cells is that they can survive with microtubules and genes for just two motor proteins, thus providing an unparalleled system to dissect microtubule and motor function within the spindle machine.
Figures




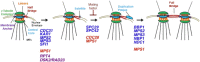



References
-
- Akhmanova A., Hoogenraad C. C., 2005. Microtubule plus-end-tracking proteins: mechanisms and functions. Curr. Opin. Cell Biol. 17: 47–54 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

