Pinceau organization in the cerebellum requires distinct functions of neurofascin in Purkinje and basket neurons during postnatal development
- PMID: 22492029
- PMCID: PMC3337041
- DOI: 10.1523/JNEUROSCI.5602-11.2012
Pinceau organization in the cerebellum requires distinct functions of neurofascin in Purkinje and basket neurons during postnatal development
Abstract
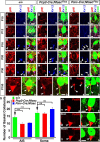
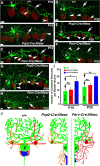
Basket axon collaterals synapse onto the Purkinje soma/axon initial segment (AIS) area to form specialized structures, the pinceau, which are critical for normal cerebellar function. Mechanistic details of how the pinceau become organized during cerebellar development are poorly understood. Loss of cytoskeletal adaptor protein Ankyrin G (AnkG) results in mislocalization of the cell adhesion molecule Neurofascin (Nfasc) at the Purkinje AIS and abnormal organization of the pinceau. Loss of Nfasc in adult Purkinje neurons leads to slow disorganization of the Purkinje AIS and pinceau morphology. Here, we used mouse conditional knock-out techniques to show that selective loss of Nfasc, specifically in Purkinje neurons during early development, prevented maturation of the AIS and resulted in loss of Purkinje neuron spontaneous activity and pinceau disorganization. Loss of Nfasc in both Purkinje and basket neurons caused abnormal basket axon collateral branching and targeting to Purkinje soma/AIS, leading to extensive pinceau disorganization, Purkinje neuron degeneration, and severe ataxia. Our studies reveal that the Purkinje Nfasc is required for AIS maturation and for maintaining stable contacts between basket axon terminals and the Purkinje AIS during pinceau organization, while the basket neuron Nfasc in combination with Purkinje Nfasc is required for proper basket axon collateral outgrowth and targeting to Purkinje soma/AIS. Thus, cerebellar pinceau organization requires coordinated mechanisms involving specific Nfasc functions in both Purkinje and basket neurons.
Figures






References
-
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. - PubMed
-
- Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98. - PubMed
-
- Bastianelli E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum. 2003;2:242–262. - PubMed
-
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
