The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors
- PMID: 22492036
- PMCID: PMC6620919
- DOI: 10.1523/JNEUROSCI.6326-11.2012
The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors
Abstract
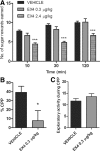
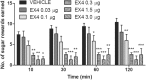
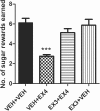
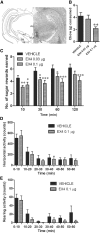
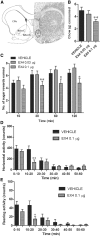
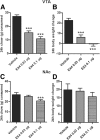
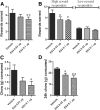
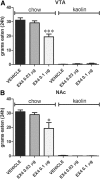
The glucagon-like peptide 1 (GLP-1) system is a recently established target for type 2 diabetes treatment. In addition to regulating glucose homeostasis, GLP-1 also reduces food intake. Previous studies demonstrate that the anorexigenic effects of GLP-1 can be mediated through hypothalamic and brainstem circuits which regulate homeostatic feeding. Here, we demonstrate an entirely novel neurobiological mechanism for GLP-1-induced anorexia in rats, involving direct effects of a GLP-1 agonist, Exendin-4 (EX4) on food reward that are exerted at the level of the mesolimbic reward system. We assessed the impact of peripheral, central, and intramesolimbic EX4 on two models of food reward: conditioned place preference (CPP) and progressive ratio operant-conditioning. Food-reward behavior was reduced in the CPP test by EX4, as rats no longer preferred an environment previously paired to chocolate pellets. EX4 also decreased motivated behavior for sucrose in a progressive ratio operant-conditioning paradigm when administered peripherally. We show that this effect is mediated centrally, via GLP-1 receptors (GLP-1Rs). GLP-1Rs are expressed in several key nodes of the mesolimbic reward system; however, their function remains unexplored. Thus we sought to determine the neurobiological substrates underlying the food-reward effect. We found that the EX4-mediated inhibition of food reward could be driven from two key mesolimbic structures-ventral tegmental area and nucleus accumbens-without inducing concurrent malaise or locomotor impairment. The current findings, that activation of central GLP-1Rs strikingly suppresses food reward/motivation by interacting with the mesolimbic system, indicate an entirely novel mechanism by which the GLP-1R stimulation affects feeding-oriented behavior.
Figures
References
-
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
