Differential association of receptor-Gβγ complexes with β-arrestin2 determines recycling bias and potential for tolerance of δ opioid receptor agonists
- PMID: 22492038
- PMCID: PMC6620903
- DOI: 10.1523/JNEUROSCI.3734-11.2012
Differential association of receptor-Gβγ complexes with β-arrestin2 determines recycling bias and potential for tolerance of δ opioid receptor agonists
Abstract
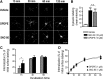
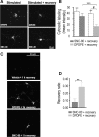
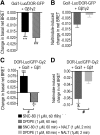
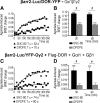
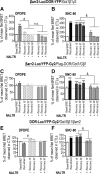
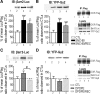
Opioid tendency to generate analgesic tolerance has been previously linked to biased internalization. Here, we assessed an alternative possibility; whether tolerance of delta opioid receptor agonists (DORs) could be related to agonist-specific recycling. A first series of experiments revealed that DOR internalization by DPDPE and SNC-80 was similar, but only DPDPE induced recycling. We then established that the non-recycling agonist SNC-80 generated acute analgesic tolerance that was absent in mice treated with DPDPE. Furthermore, both agonists stabilized different conformations, whose distinct interaction with Gβγ subunits led to different modalities of β-arrestin2 (βarr2) recruitment. In particular, bioluminescence resonance energy transfer (BRET) assays revealed that sustained activation by SNC-80 drew the receptor C terminus in close proximity of the N-terminal domain of Gγ2, causing βarr2 to interact with receptors and Gβγ subunits. DPDPE moved the receptor C-tail away from the Gβγ dimer, resulting in βarr2 recruitment to the receptor but not in the vicinity of Gγ2. These differences were associated with stable DOR-βarr2 association, poor recycling, and marked desensitization following exposure to SNC-80, while DPDPE promoted transient receptor interaction with βarr2 and effective recycling, which conferred protection from desensitization. Together, these data indicate that DORs may adopt ligand-specific conformations whose distinct recycling properties determine the extent of desensitization and are predictive of analgesic tolerance. Based on these findings, we propose that the development of functionally selective DOR ligands that favor recycling could constitute a valid strategy for the production of longer acting opioid analgesics.
Figures




References
-
- Audet N, Piñeyro G. Using BRET to detect ligand-specific conformational changes in performed signalling complexes. Methods Mol Biol. 2011;756:149–163. - PubMed
-
- Audet N, Paquin-Gobeil M, Landry-Paquet O, Schiller PW, Piñeyro G. Internalization and Src activity regulate the time course of ERK activation by delta opioid receptor ligands. J Biol Chem. 2005;280:7808–7816. - PubMed
-
- Audet N, Galés C, Archer-Lahlou E, Vallières M, Schiller PW, Bouvier M, Pineyro G. Bioluminescence resonance energy transfer assays reveal ligand-specific conformational changes within preformed signaling complexes containing delta-opioid receptors and heterotrimeric G proteins. J Biol Chem. 2008;283:15078–15088. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
