A lack of ovarian function increases neuroinflammation in aged mice
- PMID: 22492304
- PMCID: PMC3359599
- DOI: 10.1210/en.2011-1925
A lack of ovarian function increases neuroinflammation in aged mice
Abstract
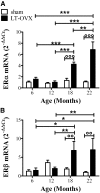
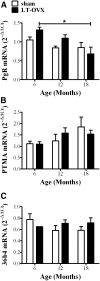
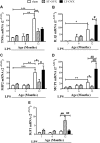
Although several lines of evidence have indicated that menopause is associated with increased susceptibility to neurological disorders, the mechanisms involved in this phenomenon remain to be elucidated. Because neuroinflammation is a common feature of a number of brain diseases, we hypothesized that the cessation of ovarian functions and the consequent decrease in estrogen receptor (ER)-mediated antiinflammatory activity may represent a trigger for postmenopausal brain dysfunctions. The aim of the present study was to investigate the effects of aging and surgical menopause on the activity of ER in neuroinflammation. The present study shows that ER genes are expressed in the hippocampus, but ER transcriptional activity decreases significantly beginning at 12 months of age in intact and ovariectomized mice. With ovariectomy, we observe an age-dependent accumulation of mRNA encoding inflammatory mediators (e.g. TNFα, IL1β, and macrophage inflammatory protein-2) and changes in the morphology of astroglia and microglia. In addition, we show that aging itself is coupled with an exaggerated response to acute inflammatory stimuli with a major accumulation of TNFα, IL1β, macrophage inflammatory protein-2, and macrophage chemoattractant protein-1 mRNA in response to lipopolysaccharide administration. The response to acute inflammatory stimuli appears to be differentially modulated by the duration of hormone deprivation in 12-month-old mice. Taken together, the present results show that aging is associated with decreased ER activity, despite continuous ER synthesis, and that age-dependent neuroinflammation is strongly influenced by hormone deprivation.
Figures



Comment in
-
Reproductive endocrinology: less estrogen, more neuroinflammation?Nat Rev Endocrinol. 2012 Apr 24;8(7):381. doi: 10.1038/nrendo.2012.65. Nat Rev Endocrinol. 2012. PMID: 22525735 No abstract available.
References
-
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. 2000. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann NY Acad Sci 908:244–254 - PubMed
-
- Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas JM, Issekutz AC, Jose PJ, Cortijo J, Morcillo EJ, Sanz MJ. 2009. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol 183:1393–1402 - PubMed
-
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. 1996. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 348:429–432 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

