COUP-TFII controls amygdala patterning by regulating neuropilin expression
- PMID: 22492355
- PMCID: PMC3317968
- DOI: 10.1242/dev.075564
COUP-TFII controls amygdala patterning by regulating neuropilin expression
Abstract
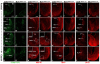
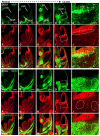
The development of the progenitor zones in the pallium, lateral ganglionic eminence (LGE) and medial ganglionic eminence (MGE) in the subpallium has been well studied; however, so far the role of the caudal ganglionic eminence (CGE), a posterior subpallial domain, in telencephalon patterning remains poorly understood. COUP-TFII, an orphan nuclear receptor, is preferentially expressed in the CGE. We generated COUP-TFII mouse mutants, using Rx-Cre (RxCre;COUP-TFII(F/F)), to study its function in telencephalon development. In these mutants, we found severe defects in the formation of the amygdala complex, including the lateral (LA), basolateral (BLA) and basomedial (BMA) amygdala nuclei. Molecular analysis provided evidence that the migration of CGE-derived Pax6(+) cells failed to settle into the BMA nucleus, owing to reduced expression of neuropilin 1 (Nrp1) and Nrp2, two semaphorin receptors that regulate neuronal cell migration and axon guidance. Our ChIP assays revealed that Nrp1 and Nrp2 genes are the direct targets of COUP-TFII in the telencephalon in vivo. Furthermore, our results showed that the coordinated development between the CGE originated subpallial population (Pax6(+) cells) and pallial populations (Tbr1(+) and Lhx2(+) cells) was essential for patterning the amygdala assembly. Our study presented novel genetic evidence that the caudal ganglionic eminence, a distinct subpallial progenitor zone, contributes cells to the basal telencephalon, such as the BMA nucleus.
Figures





References
-
- Bulchand S., Grove E. A., Porter F. D., Tole S. (2001). LIM-homeodomain gene Lhx2 regulates the formation of the cortical hem. Mech. Dev. 100, 165–175 - PubMed
-
- Bulfone A., Smiga S. M., Shimamura K., Peterson A., Puelles L., Rubenstein J. L. (1995). T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron 15, 63–78 - PubMed
-
- Bupesh M., Legaz I., Antonio Abellán A., Medina L. (2011a). Multiple telencephalic and extratelencephalic embryonic domains contribute neurons to the medial extended amygdala. J. Comp. Neurol. 519, 1505–1525 - PubMed
-
- Bupesh M., Abellán A., Medina L. (2011b). Genetic and experimental evidence supports the continuum of the central extended amygdala and a mutiple embryonic origin of its principal neurons. J. Comp. Neurol. 519, 3507–3531 - PubMed
-
- Butt S. J., Fuccillo M., Nery S., Noctor S., Kriegstein A., Corbin J. G., Fishell G. (2005). The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron 48, 591–604 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HD017379/HD/NICHD NIH HHS/United States
- HD17379/HD/NICHD NIH HHS/United States
- P01 DK059820/DK/NIDDK NIH HHS/United States
- R01 DK045641/DK/NIDDK NIH HHS/United States
- U19 DK062434/DK/NIDDK NIH HHS/United States
- R01 HD017379/HD/NICHD NIH HHS/United States
- R37 DK045641/DK/NIDDK NIH HHS/United States
- DK079638/DK/NIDDK NIH HHS/United States
- R01 NS099099/NS/NINDS NIH HHS/United States
- DK59820/DK/NIDDK NIH HHS/United States
- HL76448/HL/NHLBI NIH HHS/United States
- DK45641/DK/NIDDK NIH HHS/United States
- R01 HL076448/HL/NHLBI NIH HHS/United States
- R01 NS034661/NS/NINDS NIH HHS/United States
- DK62434/DK/NIDDK NIH HHS/United States
- P30 DK079638/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

