Estrogen receptor-α recruits P-TEFb to overcome transcriptional pausing in intron 1 of the MYB gene
- PMID: 22492511
- PMCID: PMC3401469
- DOI: 10.1093/nar/gks286
Estrogen receptor-α recruits P-TEFb to overcome transcriptional pausing in intron 1 of the MYB gene
Abstract
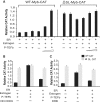
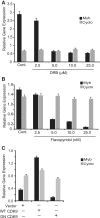
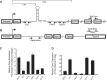
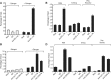
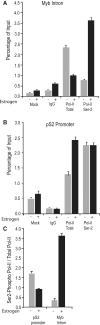
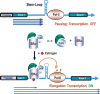
The MYB proto-oncogene is expressed in most estrogen receptor-positive (ERα(+)) breast tumors and cell lines. Expression of MYB is controlled, in breast cancer and other cell types, by a transcriptional pausing mechanism involving an attenuation site located ∼1.7 kb downstream from the transcription start site. In breast cancer cells, ligand-bound ERα binds close to, and drives transcription beyond this attenuation site, allowing synthesis of complete transcripts. However, little is known, in general, about the factors involved in relieving transcriptional attenuation, or specifically how ERα coordinates such factors to promote transcriptional elongation. Using cyclin dependent kinase 9 (CDK9) inhibitors, reporter gene assays and measurements of total and intronic MYB transcription, we show that functionally active CDK9 is required for estrogen-dependent transcriptional elongation. We further show by ChIP and co-immunoprecipitation studies that the P-TEFb complex (CDK9/CyclinT1) is recruited to the attenuation region by ligand-bound ERα, resulting in increased RNA polymerase II Ser-2 phosphorylation. These data provide new insights into MYB regulation, and given the critical roles of MYB in tumorigenesis, suggest targeting MYB elongation as potential therapeutic strategy.
Figures


References
-
- Gonda TJ, Leo P, Ramsay RG. Estrogen and MYB in breast cancer: potential for new therapies. Expert Opin. Biol. Ther. 2008;8:713–717. - PubMed
-
- Torelli G, Venturelli D, Colo A, Zanni C, Selleri L, Moretti L, Calabretta B, Torelli U. Expression of c-myb protooncogene and other cell cycle-related genes in normal and neoplastic human colonic mucosa. Cancer Res. 1987;47:5266–5269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

