Cellular responses to the metal-binding properties of metformin
- PMID: 22492524
- PMCID: PMC3357267
- DOI: 10.2337/db11-0961
Cellular responses to the metal-binding properties of metformin
Abstract
In recent decades, the antihyperglycemic biguanide metformin has been used extensively in the treatment of type 2 diabetes, despite continuing uncertainty over its direct target. In this article, using two independent approaches, we demonstrate that cellular actions of metformin are disrupted by interference with its metal-binding properties, which have been known for over a century but little studied by biologists. We demonstrate that copper sequestration opposes known actions of metformin not only on AMP-activated protein kinase (AMPK)-dependent signaling, but also on S6 protein phosphorylation. Biguanide/metal interactions are stabilized by extensive π-electron delocalization and by investigating analogs of metformin; we provide evidence that this intrinsic property enables biguanides to regulate AMPK, glucose production, gluconeogenic gene expression, mitochondrial respiration, and mitochondrial copper binding. In contrast, regulation of S6 phosphorylation is prevented only by direct modification of the metal-liganding groups of the biguanide structure, supporting recent data that AMPK and S6 phosphorylation are regulated independently by biguanides. Additional studies with pioglitazone suggest that mitochondrial copper is targeted by both of these clinically important drugs. Together, these results suggest that cellular effects of biguanides depend on their metal-binding properties. This link may illuminate a better understanding of the molecular mechanisms enabling antihyperglycemic drug action.
Figures
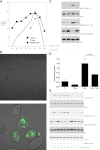
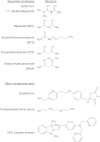




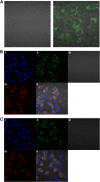
References
-
- Slotta KH, Tschesche R. Über Biguanide, II.: Die blutzucker-senkende Wirkung der Biguanide. Ber Dtsch Chem Ges 1929;62:1398–1405
-
- Hawgood BJ. Karl Heinrich Slotta (1895–1987) biochemist: snakes, pregnancy and coffee. Toxicon 2001;39:1277–1282 - PubMed
-
- Shapiro SL, Parrino VA, Freedman L. Hypoglycemic agents III. N-Alkyl- and arylalkylbiguanides. J Am Chem Soc 1959;81:3728–3736
-
- El-Mir M-Y, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 2000;275:223–228 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

