Tuning electrical conduction along endothelial tubes of resistance arteries through Ca(2+)-activated K(+) channels
- PMID: 22492531
- PMCID: PMC3467972
- DOI: 10.1161/CIRCRESAHA.111.262592
Tuning electrical conduction along endothelial tubes of resistance arteries through Ca(2+)-activated K(+) channels
Abstract
Rationale: Electrical conduction through gap junction channels between endothelial cells of resistance vessels is integral to blood flow control. Small and intermediate-conductance Ca(2+)-activated K(+) channels (SK(Ca)/IK(Ca)) initiate electrical signals in endothelial cells, but it is unknown whether SK(Ca)/IK(Ca) activation alters signal transmission along the endothelium.
Objective: We tested the hypothesis that SK(Ca)/IK(Ca) activity regulates electrical conduction along the endothelium of resistance vessels.
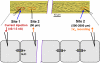
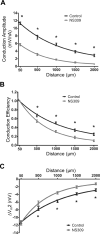
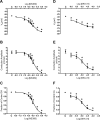
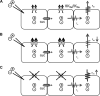
Methods and results: Freshly isolated endothelial cell tubes (60 μm wide; 1-3 mm long; cell length, ≈35 μm) from mouse skeletal muscle feed (superior epigastric) arteries were studied using dual intracellular microelectrodes. Current was injected (±0.1-3 nA) at site 1 while recording membrane potential (V(m)) at site 2 (separation distance=50-2000 μm). SK(Ca)/IK(Ca) activation (NS309, 1 μmol/L) reduced the change in V(m) along endothelial cell tubes by ≥50% and shortened the electrical length constant (λ) from 1380 to 850 μm (P<0.05) while intercellular dye transfer (propidium iodide) was maintained. Activating SK(Ca)/IK(Ca) with acetylcholine or SKA-31 also reduced electrical conduction. These effects of SK(Ca)/IK(Ca) activation persisted when hyperpolarization (>30 mV) was prevented with 60 mmol/L [K(+)](o). Conversely, blocking SK(Ca)/IK(Ca) (apamin+charybdotoxin) depolarized cells by ≈10 mV and enhanced electrical conduction (ie, changes in V(m)) by ≈30% (P<0.05).
Conclusions: These findings illustrate a novel role for SK(Ca)/IK(Ca) activity in tuning electrical conduction along the endothelium of resistance vessels by governing signal dissipation through changes in membrane resistance. Voltage-insensitive ion channels can thereby tune intercellular electrical signaling independent from gap junction channels.
Figures





References
-
- Beny JL. Information networks in the arterial wall. News Physiol Sci. 1999;14:68–73. - PubMed
-
- Schmidt VJ, Wolfle SE, Boettcher M, de Wit C. Gap junctions synchronize vascular tone within the microcirculation. Pharmacol Rep. 2008;60:68–74. - PubMed
-
- Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. - PubMed
-
- Looft-Wilson RC, Payne GW, Segal S. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol. 2004;97:1152–1158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

