Essential role of interleukin-6 in post-stroke angiogenesis
- PMID: 22492561
- PMCID: PMC3359750
- DOI: 10.1093/brain/aws075
Essential role of interleukin-6 in post-stroke angiogenesis
Abstract
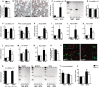
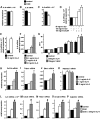
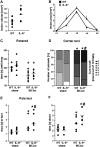
Ambivalent effects of interleukin-6 on the pathogenesis of ischaemic stroke have been reported. However, to date, the long-term actions of interleukin-6 after stroke have not been investigated. Here, we subjected interleukin-6 knockout (IL-6(-/-)) and wild-type control mice to mild brain ischaemia by 30-min filamentous middle cerebral artery occlusion/reperfusion. While ischaemic tissue damage was comparable at early time points, IL-6(-/-) mice showed significantly increased chronic lesion volumes as well as worse long-term functional outcome. In particular, IL-6(-/-) mice displayed an impaired angiogenic response to brain ischaemia with reduced numbers of newly generated endothelial cells and decreased density of perfused microvessels along with lower absolute regional cerebral blood flow and reduced vessel responsivity in ischaemic striatum at 4 weeks. Similarly, the early genomic activation of angiogenesis-related gene networks was strongly reduced and the ischaemia-induced signal transducer and activator of transcription 3 activation observed in wild-type mice was almost absent in IL-6(-/-) mice. In addition, systemic neoangiogenesis was impaired in IL-6(-/-) mice. Transplantation of interleukin-6 competent bone marrow into IL-6(-/-) mice (IL-6(chi)) did not rescue interleukin-6 messenger RNA expression or the early transcriptional activation of angiogenesis after stroke. Accordingly, chronic stroke outcome in IL-6(chi) mice recapitulated the major effects of interleukin-6 deficiency on post-stroke regeneration with significantly enhanced lesion volumes and reduced vessel densities. Additional in vitro experiments yielded complementary evidence, which showed that after stroke resident brain cells serve as the major source of interleukin-6 in a self-amplifying network. Treatment of primary cortical neurons, mixed glial cultures or immortalized brain endothelia with interleukin 6-induced robust interleukin-6 messenger RNA transcription in each case, whereas oxygen-glucose deprivation did not. However, oxygen-glucose deprivation of organotypic brain slices resulted in strong upregulation of interleukin-6 messenger RNA along with increased transcription of key angiogenesis-associated genes. In conclusion, interleukin-6 produced locally by resident brain cells promotes post-stroke angiogenesis and thereby affords long-term histological and functional protection.
Figures



References
-
- Acalovschi D, Wiest T, Hartmann M, Farahmi M, Mansmann U, Auffarth GU, et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34:1864–9. - PubMed
-
- Andjelkovic AV, Stamatovic SM, Keep RF. The protective effects of preconditioning on cerebral endothelial cells in vitro. J Cereb Blood Flow Metab. 2003;23:1348–55. - PubMed
-
- Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–73. - PubMed
-
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–57. - PubMed

