Evaluation of influenza virus A/H3N2 and B vaccines on the basis of cross-reactivity of postvaccination human serum antibodies against influenza viruses A/H3N2 and B isolated in MDCK cells and embryonated hen eggs
- PMID: 22492743
- PMCID: PMC3370437
- DOI: 10.1128/CVI.05726-11
Evaluation of influenza virus A/H3N2 and B vaccines on the basis of cross-reactivity of postvaccination human serum antibodies against influenza viruses A/H3N2 and B isolated in MDCK cells and embryonated hen eggs
Abstract
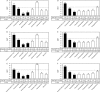
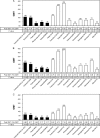
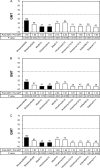
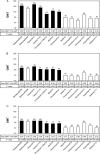
The vaccine strains against influenza virus A/H3N2 for the 2010-2011 season and influenza virus B for the 2009-2010 and 2010-2011 seasons in Japan are a high-growth reassortant A/Victoria/210/2009 (X-187) strain and an egg-adapted B/Brisbane/60/2008 (Victoria lineage) strain, respectively. Hemagglutination inhibition (HI) tests with postinfection ferret antisera indicated that the antisera raised against the X-187 and egg-adapted B/Brisbane/60/2008 vaccine production strains poorly inhibited recent epidemic isolates of MDCK-grown A/H3N2 and B/Victoria lineage viruses, respectively. The low reactivity of the ferret antisera may be attributable to changes in the hemagglutinin (HA) protein of production strains during egg adaptation. To evaluate the efficacy of A/H3N2 and B vaccines, the cross-reactivities of postvaccination human serum antibodies against A/H3N2 and B/Victoria lineage epidemic isolates were assessed by a comparison of the geometric mean titers (GMTs) of HI and neutralization (NT) tests. Serum antibodies elicited by the X-187 vaccine had low cross-reactivity to both MDCK- and egg-grown A/H3N2 isolates by HI test and narrow cross-reactivity by NT test in all age groups. On the other hand, the GMTs to B viruses detected by HI test were below the marginal level, so the cross-reactivity was assessed by NT test. The serum neutralizing antibodies elicited by the B/Brisbane/60/2008 vaccine reacted well with egg-grown B viruses but exhibited remarkably low reactivity to MDCK-grown B viruses. The results of these human serological studies suggest that the influenza A/H3N2 vaccine for the 2010-2011 season and B vaccine for the 2009-2010 and 2010-2011 seasons may possess insufficient efficacy and low efficacy, respectively.
Figures


Similar articles
-
Evaluation of a qualified MDCK cell line for virus isolation to develop cell-based influenza vaccine viruses with appropriate antigenicity.Vaccine. 2024 Oct 3;42(23):126242. doi: 10.1016/j.vaccine.2024.126242. Epub 2024 Aug 29. Vaccine. 2024. PMID: 39213922
-
Neutralizing Antibody Responses to Antigenically Drifted Influenza A(H3N2) Viruses among Children and Adolescents following 2014-2015 Inactivated and Live Attenuated Influenza Vaccination.Clin Vaccine Immunol. 2016 Oct 4;23(10):831-839. doi: 10.1128/CVI.00297-16. Print 2016 Oct. Clin Vaccine Immunol. 2016. PMID: 27558294 Free PMC article.
-
Evaluation of immune responses to inactivated influenza vaccines prepared in embryonated chicken eggs and MDCK cells in a mouse model.Dev Biol Stand. 1999;98:53-63; discussion 73-4. Dev Biol Stand. 1999. PMID: 10494959
-
H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation.Hum Vaccin Immunother. 2018;14(8):1840-1847. doi: 10.1080/21645515.2018.1462639. Epub 2018 May 14. Hum Vaccin Immunother. 2018. PMID: 29641358 Free PMC article. Review.
-
Universal influenza virus vaccines and therapeutics: where do we stand with influenza B virus?Curr Opin Immunol. 2018 Aug;53:45-50. doi: 10.1016/j.coi.2018.04.002. Epub 2018 Apr 17. Curr Opin Immunol. 2018. PMID: 29677684 Free PMC article. Review.
Cited by
-
Retrospective Assessment of the Antigenic Similarity of Egg-Propagated and Cell Culture-Propagated Reference Influenza Viruses as Compared with Circulating Viruses across Influenza Seasons 2002-2003 to 2017-2018.Int J Environ Res Public Health. 2020 Jul 28;17(15):5423. doi: 10.3390/ijerph17155423. Int J Environ Res Public Health. 2020. PMID: 32731417 Free PMC article.
-
Subtype H3N2 Influenza A Viruses: An Unmet Challenge in the Western Pacific.Vaccines (Basel). 2022 Jan 12;10(1):112. doi: 10.3390/vaccines10010112. Vaccines (Basel). 2022. PMID: 35062773 Free PMC article. Review.
-
Immunogenicity and safety of an egg-based inactivated quadrivalent influenza vaccine (GC3110A) versus two inactivated trivalent influenza vaccines with alternate B strains: A phase Ⅲ randomized clinical trial in adults.Hum Vaccin Immunother. 2019;15(3):710-716. doi: 10.1080/21645515.2018.1536589. Epub 2018 Nov 15. Hum Vaccin Immunother. 2019. PMID: 30396317 Free PMC article. Clinical Trial.
-
Improved immunogenicity of individual influenza vaccine components delivered with a novel dissolving microneedle patch stable at room temperature.Drug Deliv Transl Res. 2015 Aug;5(4):360-71. doi: 10.1007/s13346-015-0228-0. Drug Deliv Transl Res. 2015. PMID: 25895053 Free PMC article.
-
H3N2 Mismatch of 2014-15 Northern Hemisphere Influenza Vaccines and Head-to-head Comparison between Human and Ferret Antisera derived Antigenic Maps.Sci Rep. 2015 Oct 16;5:15279. doi: 10.1038/srep15279. Sci Rep. 2015. PMID: 26472175 Free PMC article.
References
-
- Ansaldi F, et al. 2012. Intanza(®) 15 intradermal influenza vaccine elicits cross-reactive antibody responses against heterologous A(H3N2) influenza viruses. Vaccine 30:2908–2913 - PubMed
-
- Arden NH, et al. 1986. Safety and immunogenicity of a 45-microgram supplemental dose of inactivated split-virus influenza B vaccine in the elderly. J. Infect. Dis. 153:805–806 - PubMed
-
- Beare AS, Schild GC, Craig JW. 1975. Trials in man with live recombinants made from A/PR/8/34 (H0 N1) and wild H3 N2 influenza viruses. Lancet ii:729–732 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

