Complex N-glycans are essential, but core 1 and 2 mucin O-glycans, O-fucose glycans, and NOTCH1 are dispensable, for mammalian spermatogenesis
- PMID: 22492969
- PMCID: PMC3386147
- DOI: 10.1095/biolreprod.111.098103
Complex N-glycans are essential, but core 1 and 2 mucin O-glycans, O-fucose glycans, and NOTCH1 are dispensable, for mammalian spermatogenesis
Abstract
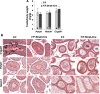
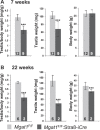
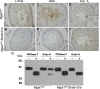
To identify roles in spermatogenesis for major subclasses of N- and O-glycans and Notch signaling, male mice carrying floxed C1galt1, Pofut1, Notch1 or Mgat1 alleles and a testis-specific Cre recombinase transgene were generated. T-synthase (C1GALT1) transfers Gal to generate core 1 and core 2 mucin O-glycans; POFUT1 transfers O-fucose to particular epidermal growth factor-like repeats and is essential for canonical Notch signaling; and MGAT1 (GlcNAcT-I) transfers GlcNAc to initiate hybrid and complex N-glycan synthesis. Cre recombinase transgenes driven by various promoters were investigated, including Stra8-iCre expressed in spermatogonia, Sycp1-Cre expressed in spermatocytes, Prm1-Cre expressed in spermatids, and AMH-Cre expressed in Sertoli cells. All Cre transgenes deleted floxed alleles, but efficiencies varied widely. Stra8-iCre was the most effective, deleting floxed Notch1 and Mgat1 alleles with 100% efficiency and floxed C1galt1 and Pofut1 alleles with ~80% efficiency, based on transmission of deleted alleles. Removal of C1galt1, Pofut1, or Notch1 in spermatogonia had no effect on testicular weight, histology, or fertility. However, males in which the synthesis of complex N-glycans was blocked by deletion of Mgat1 in spermatogonia did not produce sperm. Spermatogonia, spermatocytes, and spermatids were generated, but most spermatids formed giant multinucleated cells or symplasts, and apoptosis was increased. Therefore, although core 1 and 2 mucin O-glycans, NOTCH1, POFUT1, O-fucose glycans, and Notch signaling are dispensable, MGAT1 and complex N-glycans are essential for spermatogenesis.
Figures





References
-
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech 2010; 73: 241 278 - PubMed
-
- Jones CJ, Morrison CA, Stoddart RW. Histochemical analysis of rat testicular glycoconjugates. 2. Beta-galactosyl residues in O- and N-linked glycans in seminiferous tubules. Histochem J 1992; 24: 327 336 - PubMed
-
- Jones CJ, Morrison CA, Stoddart RW. Histochemical analysis of rat testicular glycoconjugates. 1. Subsets of N-linked saccharides in seminiferous tubules. Histochem J 1992; 24: 319 326 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

