Trm112 is required for Bud23-mediated methylation of the 18S rRNA at position G1575
- PMID: 22493060
- PMCID: PMC3372273
- DOI: 10.1128/MCB.06623-11
Trm112 is required for Bud23-mediated methylation of the 18S rRNA at position G1575
Abstract
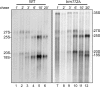
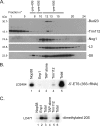
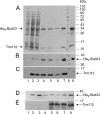
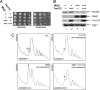
Posttranscriptional and posttranslational modification of macromolecules is known to fine-tune their functions. Trm112 is unique, acting as an activator of both tRNA and protein methyltransferases. Here we report that in Saccharomyces cerevisiae, Trm112 is required for efficient ribosome synthesis and progression through mitosis. Trm112 copurifies with pre-rRNAs and with multiple ribosome synthesis trans-acting factors, including the 18S rRNA methyltransferase Bud23. Consistent with the known mechanisms of activation of methyltransferases by Trm112, we found that Trm112 interacts directly with Bud23 in vitro and that it is required for its stability in vivo. Consequently, trm112Δ cells are deficient for Bud23-mediated 18S rRNA methylation at position G1575 and for small ribosome subunit formation. Bud23 failure to bind nascent preribosomes activates a nucleolar surveillance pathway involving the TRAMP complexes, leading to preribosome degradation. Trm112 is thus active in rRNA, tRNA, and translation factor modification, ideally placing it at the interface between ribosome synthesis and function.
Figures





References
-
- Brunger AT. 2007. Version 1.2 of the Crystallography and NMR system. Nat. Protoc. 2: 2728– 2733 - PubMed
-
- Chern MK, et al. 2009. Single-step protein purification by back flush in ion exchange chromatography. Anal. Biochem. 392: 174– 176 - PubMed
-
- Decatur WA, Fournier MJ. 2002. rRNA modifications and ribosome function. Trends Biochem. Sci. 27: 344– 351 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
