Protection from bacterial-toxin-induced apoptosis in macrophages requires the lipogenic transcription factor sterol regulatory element binding protein 1a
- PMID: 22493063
- PMCID: PMC3372266
- DOI: 10.1128/MCB.06294-11
Protection from bacterial-toxin-induced apoptosis in macrophages requires the lipogenic transcription factor sterol regulatory element binding protein 1a
Abstract
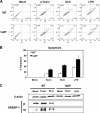
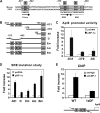
Sterol regulatory element binding protein (SREBP) transcription factors activate genes of lipid metabolism, but recent studies indicate they also activate genes involved in other physiologic processes, suggesting that SREPBs have evolved to connect lipid metabolism with diverse physiologic responses. There are three major mammalian SREBPs, and the 1a isoform is specifically expressed at very high levels in macrophages, where a recent study showed that it couples lipid synthesis to the proinflammatory phase of the innate immune response. In the present study, we show that loss of SREBP-1a also results in an increase in apoptosis after exposure to bacterial pore-forming toxins and we show this is a result of a selective reduction in the expression of the gene coding for the antiapoptotic factor apoptosis inhibitor 6 (Api6). Additional studies demonstrate that SREBP-1a specifically activates the Api6 gene through a binding site in its proximal promoter, thus establishing the Api6 gene as a newly identified SREBP-1a target gene.
Figures





References
-
- Aderem A, Underhill DM. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17: 593– 623 - PubMed
-
- Earnshaw WC, Martins LM, Kaufmann SH. 1999. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68: 383– 424 - PubMed
-
- Gagnon E, et al. 2002. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110: 119– 131 - PubMed
-
- Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126: 1135– 1145 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
