Engagement of β-arrestin by transactivated insulin-like growth factor receptor is needed for V2 vasopressin receptor-stimulated ERK1/2 activation
- PMID: 22493236
- PMCID: PMC3340024
- DOI: 10.1073/pnas.1112422109
Engagement of β-arrestin by transactivated insulin-like growth factor receptor is needed for V2 vasopressin receptor-stimulated ERK1/2 activation
Abstract
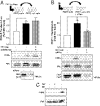
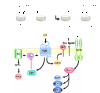
G protein-coupled receptors (GPCRs) have been shown to activate the mitogen-activated protein kinases, ERK1/2, through both G protein-dependent and -independent mechanisms. Here, we describe a G protein-independent mechanism that unravels an unanticipated role for β-arrestins. Stimulation of the V2 vasopressin receptor (V2R) in cultured cells or in vivo in rat kidney medullar collecting ducts led to the activation of ERK1/2 through the metalloproteinase-mediated shedding of a factor activating the insulin-like growth factor receptor (IGFR). This process was found to be both Src- and β-arrestin-dependent. Whereas Src was found to act upstream of the metalloproteinase activation and be required for the release of the IGFR-activating factor, β-arrestins were found to act downstream of the IGFR transactivation. Unexpectedly, the engagement of β-arrestins by the IGFR but not by the V2R was needed to promote the vasopressin-stimulated ERK1/2 activation, indicating that a pool of β-arrestins distinct from those β-arrestins recruited to the V2R acts downstream of the receptor tyrosine kinase to activate ERK1/2. Such a dual site of action for β-arrestins helps explain the pleiotropic actions of this scaffolding protein. Given the role that V2R-stimulated ERK1/2 plays in kidney cell proliferation, this transactivation mechanism may have important implications for renal pathophysiology. Still, the role of β-arrestins downstream of a transactivation event is not limited to the V2R, because we observed a similar involvement for an unrelated GPCR (the platelet-activating factor receptor), indicating that it may be a general mechanism shared among GPCRs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





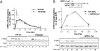

References
-
- Charest PG, Bouvier M. Palmitoylation of the V2 vasopressin receptor carboxyl tail enhances beta-arrestin recruitment leading to efficient receptor endocytosis and ERK1/2 activation. J Biol Chem. 2003;278:41541–41551. - PubMed
-
- Tohgo A, et al. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258–6267. - PubMed
-
- Charest PG, Oligny-Longpré G, Bonin H, Azzi M, Bouvier M. The V2 vasopressin receptor stimulates ERK1/2 activity independently of heterotrimeric G protein signalling. Cell Signal. 2007;19:32–41. - PubMed
-
- Werry TD, Sexton PM, Christopoulos A. “Ins and outs” of seven-transmembrane receptor signalling to ERK. Trends Endocrinol Metab. 2005;16:26–33. - PubMed
-
- Wetzker R, Böhmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol. 2003;4:651–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

