Bax regulates primary necrosis through mitochondrial dynamics
- PMID: 22493254
- PMCID: PMC3340068
- DOI: 10.1073/pnas.1201608109
Bax regulates primary necrosis through mitochondrial dynamics
Abstract
The defining event in apoptosis is mitochondrial outer membrane permeabilization (MOMP), allowing apoptogen release. In contrast, the triggering event in primary necrosis is early opening of the inner membrane mitochondrial permeability transition pore (mPTP), precipitating mitochondrial dysfunction and cessation of ATP synthesis. Bcl-2 proteins Bax and Bak are the principal activators of MOMP and apoptosis. Unexpectedly, we find that deletion of Bax and Bak dramatically reduces necrotic injury during myocardial infarction in vivo. Triple knockout mice lacking Bax/Bak and cyclophilin D, a key regulator of necrosis, fail to show further reduction in infarct size over those deficient in Bax/Bak. Absence of Bax/Bak renders cells resistant to mPTP opening and necrosis, effects confirmed in isolated mitochondria. Reconstitution of these cells or mitochondria with wild-type Bax, or an oligomerization-deficient mutant that cannot support MOMP and apoptosis, restores mPTP opening and necrosis, implicating distinct mechanisms for Bax-regulated necrosis and apoptosis. Both forms of Bax restore mitochondrial fusion in Bax/Bak-null cells, which otherwise exhibit fragmented mitochondria. Cells lacking mitofusin 2 (Mfn2), which exhibit similar fusion defects, are protected to the same extent as Bax/Bak-null cells. Conversely, restoration of fused mitochondria through inhibition of fission potentiates mPTP opening in the absence of Bax/Bak or Mfn2, indicating that the fused state itself is critical. These data demonstrate that Bax-driven fusion lowers the threshold for mPTP opening and necrosis. Thus, Bax and Bak play wider roles in cell death than previously appreciated and may be optimal therapeutic targets for diseases that involve both forms of cell death.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
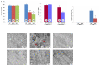
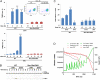

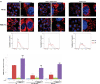
References
-
- Zong WX, Thompson CB. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. - PubMed
-
- Golstein P, Kroemer G. Cell death by necrosis: Towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. - PubMed
-
- Kung G, Konstantinidis K, Kitsis RN. Programmed necrosis, not apoptosis, in the heart. Circ Res. 2011;108:1017–1036. - PubMed
-
- Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140:798–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 HL095929/HL/NHLBI NIH HHS/United States
- 5R01HL059888-12/HL/NHLBI NIH HHS/United States
- 5P30CA013330-39/CA/NCI NIH HHS/United States
- R01 HL060665/HL/NHLBI NIH HHS/United States
- T32 AG023475/AG/NIA NIH HHS/United States
- 5P60DK020541-34/DK/NIDDK NIH HHS/United States
- 4R00HL095929-02/HL/NHLBI NIH HHS/United States
- 5R37HL054598-16/HL/NHLBI NIH HHS/United States
- P60 DK020541/DK/NIDDK NIH HHS/United States
- 5R01HL60665-13/HL/NHLBI NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- R37 HL054598/HL/NHLBI NIH HHS/United States
- R01 HL098481/HL/NHLBI NIH HHS/United States
- R01 HL059888/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

