Y265C DNA polymerase beta knockin mice survive past birth and accumulate base excision repair intermediate substrates
- PMID: 22493258
- PMCID: PMC3340078
- DOI: 10.1073/pnas.1200800109
Y265C DNA polymerase beta knockin mice survive past birth and accumulate base excision repair intermediate substrates
Abstract
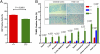
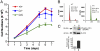
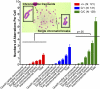
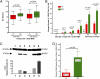
DNA is susceptible to damage by a wide variety of chemical agents that are generated either as byproducts of cellular metabolism or exposure to man-made and harmful environments. Therefore, to maintain genomic integrity, having reliable DNA repair systems is important. DNA polymerase β is known to be a key player in the base excision repair pathway, and mice devoid of DNA polymerase beta do not live beyond a few hours after birth. In this study, we characterized mice harboring an impaired pol β variant. This Y265C pol β variant exhibits slow DNA polymerase activity but WT lyase activity and has been shown to be a mutator polymerase. Mice expressing Y265C pol β are born at normal Mendelian ratios. However, they are small, and 60% die within a few hours after birth. Slow proliferation and significantly increased levels of cell death are observed in many organs of the E14 homozygous embryos compared with WT littermates. Mouse embryo fibroblasts prepared from the Y265C pol β embryos proliferate at a rate slower than WT cells and exhibit a gap-filling deficiency during base excision repair. As a result of this, chromosomal aberrations and single- and double-strand breaks are present at significantly higher levels in the homozygous mutant versus WT mouse embryo fibroblasts. This is study in mice is unique in that two enzymatic activities of pol β have been separated; the data clearly demonstrate that the DNA polymerase activity of pol β is essential for survival and genome stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- De Bont R, van Larebeke N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis. 2004;19:169–185. - PubMed
-
- Wallace SS. Biological consequences of free radical-damaged DNA bases. Free Radic Biol Med. 2002;33:1–14. - PubMed
-
- Barnes DE, Lindahl T. Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet. 2004;38:445–476. - PubMed
-
- Loeb LA, Monnat RJ., Jr DNA polymerases and human disease. Nat Rev Genet. 2008;9:594–604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

