Role of autophagy in histone deacetylase inhibitor-induced apoptotic and nonapoptotic cell death
- PMID: 22493260
- PMCID: PMC3340088
- DOI: 10.1073/pnas.1204429109
Role of autophagy in histone deacetylase inhibitor-induced apoptotic and nonapoptotic cell death
Abstract
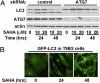
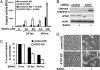
Autophagy is a cellular catabolic pathway by which long-lived proteins and damaged organelles are targeted for degradation. Activation of autophagy enhances cellular tolerance to various stresses. Recent studies indicate that a class of anticancer agents, histone deacetylase (HDAC) inhibitors, can induce autophagy. One of the HDAC inhibitors, suberoylanilide hydroxamic acid (SAHA), is currently being used for treating cutaneous T-cell lymphoma and under clinical trials for multiple other cancer types, including glioblastoma. Here, we show that SAHA increases the expression of the autophagic factor LC3, and inhibits the nutrient-sensing kinase mammalian target of rapamycin (mTOR). The inactivation of mTOR results in the dephosphorylation, and thus activation, of the autophagic protein kinase ULK1, which is essential for autophagy activation during SAHA treatment. Furthermore, we show that the inhibition of autophagy by RNAi in glioblastoma cells results in an increase in SAHA-induced apoptosis. Importantly, when apoptosis is pharmacologically blocked, SAHA-induced nonapoptotic cell death can also be potentiated by autophagy inhibition. Overall, our findings indicate that SAHA activates autophagy via inhibiting mTOR and up-regulating LC3 expression; autophagy functions as a prosurvival mechanism to mitigate SAHA-induced apoptotic and nonapoptotic cell death, suggesting that targeting autophagy might improve the therapeutic effects of SAHA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

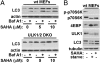

References
-
- Khan O, La Thangue NB. HDAC inhibitors in cancer biology: Emerging mechanisms and clinical applications. Immunol Cell Biol. 2012;90:85–94. - PubMed
-
- Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90. - PubMed
-
- Rosato RR, Almenara JA, Dai Y, Grant S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther. 2003;2:1273–1284. - PubMed
-
- Emanuele S, Lauricella M, Tesoriere G. Histone deacetylase inhibitors: Apoptotic effects and clinical implications (Review) Int J Oncol. 2008;33:637–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

